Imidazolothiazole compounds for the treatment of disease
A compound, independent technology, applied in metabolic diseases, skin diseases, sensory diseases, etc., can solve problems such as RTK damage deactivation
- Summary
- Abstract
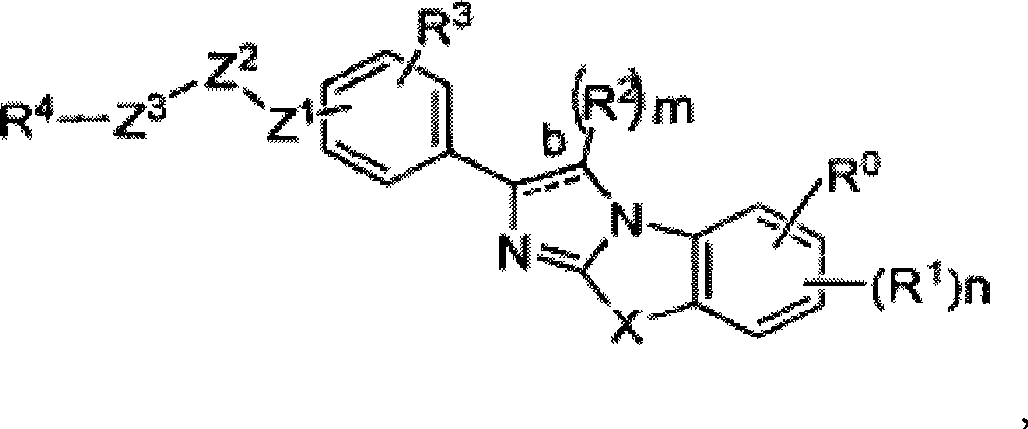
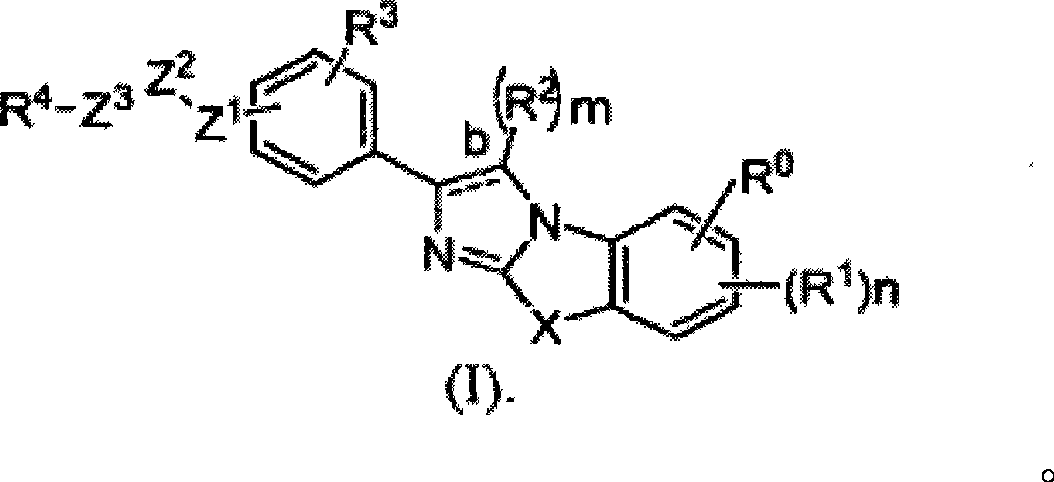
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
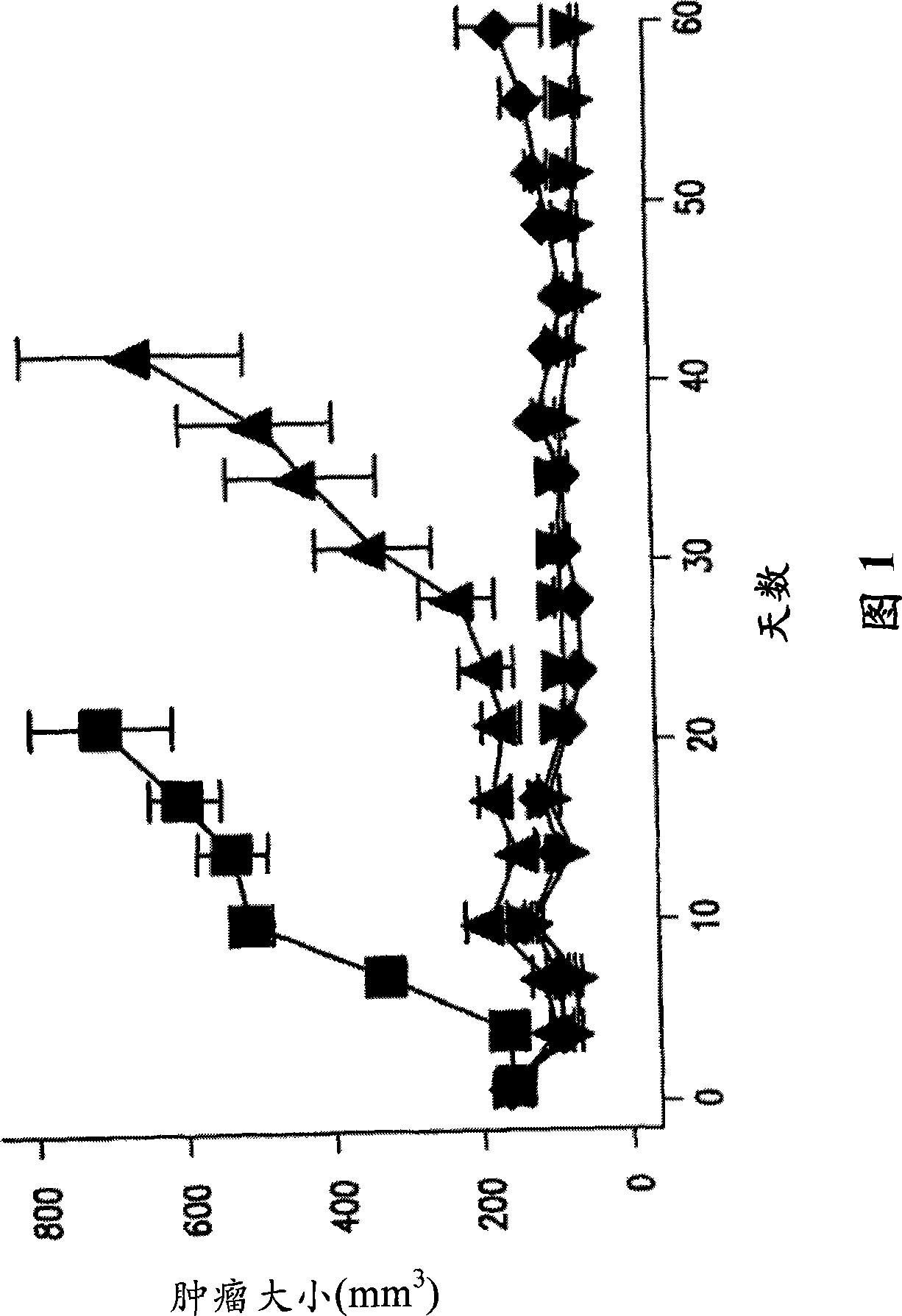
Image
Examples
preparation example Construction
[0470] G. Compound Preparation
[0471] The starting materials of the synthetic examples provided by the present invention can be purchased through commercial channels, or obtained by literature methods (such as March Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, (1992) 4th Ed.; Wiley Interscience, New York ). All commercially available compounds were used without further purification unless otherwise stated. CDCl 3 (99.8% D, Cambridge Isotope Laboratories) was used in all experiments shown. Proton ( 1 h) The nuclear magnetic resonance (NMR) spectrum was recorded by a Bruker Avance 300 MHz NMR spectrometer. The list of notable peaks generally includes: number of protons, and multiplicity (s, singlet; d, doublet; t, triplet; q, quadruplet; m, multiplet; brs, broad singlet), chemical shift is It is described in parts per million (δ) relative to tetramethylsilane. Low resolution mass spectra (MS) were obtained as electrospray ionization (ESI) mass spectr...
Embodiment 1
[0531] Example 1: N-(5-tert-butyl-isoxazol-3-yl)-N'-imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl} Preparation of urea [compound A1]
[0532] A. Preparation of intermediate 2-(4-nitrophenyl)imidazo[2,1-b][1,3]benzothiazole. Dissolve 2-aminobenzothiazole (751 mg, 5 mmol) and 2-bromo-4'-nitroacetophenone (1.22 g, 5 mmol) in ethanol, and heat to reflux overnight. The solution was then cooled at room temperature for 24 hours. The precipitate was collected by filtration, washed with methanol and dried in vacuo.
[0533] B. Preparation of 2-(4-amino-phenyl)imidazo[2,1-b][1,3]benzothiazole. The intermediate from step A (428 mg, 1.5 mmol) was prepared as a suspension in isopropanol, to which iron powder (419 mg, 7.5 mmol) was added. The suspension was heated to reflux overnight with vigorous stirring. Completion of the reaction was confirmed by LCMS. 1N HCl was added to the mixture and allowed to cool to room temperature. The precipitate was collected by filtration and washed wit...
Embodiment 2
[0540] Example 2: 1-(5-tert-butyl-isoxazol-3-yl)-3-[4-(7-morpholin-4-yl-benzo[d]imidazo[2,1-b ]thiazol-2-yl)-phenyl]-urea preparation; [compound A6]
[0541] A. Preparation of intermediate 6-morpholin-4-yl-benzothiazol-2-amine: NH 4 SCN (2.28 g, 30 mmol) was added in small portions to a solution of 4-N-morpholinoaniline (1.78 g, 10 mmol) in acetic acid (20 mL). After stirring the mixture for 30 minutes, a solution of bromine in acetic acid (1.6 g in 5 mL) was added to the mixture, and the mixture was stirred at room temperature overnight. The mixture was then heated at 90 °C for 30 min, followed by cooling and washing with saturated NaHCO 3 Neutralize, then use CH 2 Cl 2 Extract 3 times. The combined organic phases were washed with MgSO 4 Dry and concentrate to dryness. Add 30 mL of 10% HCI to the residue, wash with saturated NaHCO 3 Neutralization afforded a tan solid (1.541 g, 66%).
[0542] B. Preparation of intermediate 7-morpholin-4-yl-2-(4-nitro-phenyl)-imidazo[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com