Application of N-(5-bromo-2-methoxyphenyl)benzoimidazothiazole carboxamide
A technology of methoxyphenyl and methylbenzene, which is applied in the application field of thiazole compounds, can solve the serious problems of caries prevention, achieve the inhibition of the formation of streptococcus mutans planktonic cells and biofilms, and have broad application prospects. strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1: Preparation of N-(5-bromo-2-methoxyphenyl)-3-methylbenzo[4,5]imidazo[2,1-b]thiazole-2-carboxamide
[0016] (1) Preparation of 2-mercaptobenzimidazole
[0017] Place o-phenylenediamine (21.61g, 0.20mol), carbon disulfide (18.24g, 0.24mol), sodium carbonate (14.84g, 0.14mol) and 300mL of water in a 500mL eggplant-shaped bottle, and heat to reflux for 7 hours. Cool to room temperature, suction filter, and dry to obtain 29.4 g of white solid, yield 98.0%.
[0018] m.p.290-292℃(lit.:303-305℃), ESI-MS(m / z):151.1([M+H]+);
[0019] IR(KBr):υ3441,3153,3114,2979,2877,1618,1513,1467,1215;
[0020] 1 H-NMR (400MHz, DMSO): δ7.10~7.15 (m, 4H), 12.51 (s, 2H).
[0021] (2) Preparation of ethyl 2-((1H-benzo[d]imidazol-2-yl)mercapto)-3-oxybutanoate
[0022] Put 2-mercaptobenzimidazole (18.00g, 0.12mol), sodium bicarbonate (30.24g, 0.36mol), ethyl 2-chloroacetoacetate (22.96g, 0.14mol) and 200mL of ethanol in a 1000mL eggplant-shaped flask , after stirring at room tempera...
Embodiment 2
[0048] Embodiment 2: the cultivation of Streptococcus mutans
[0049] (1) The medium for cultivating Streptococcus mutans is Brain Heart Infusion medium (brand OXOID, product number CM1135). The main components of the medium are Brain infusion solids 12.5g / L, Beef heartinfusion solids 5.0g / L , Proteose peptone 10.0g / L, Glucose 2.0g / L, Sodiumchloride 5.0g / L, Di-sodium phosphate 2.5g / L, pH 7.4±0.2. If it needs to be configured into a solid, 15g / L of agar powder needs to be added. Sterilize at 115°C for 30 minutes and cool down for use.
[0050] (2) The medium for cultivating Streptococcus mutans biofilm is brain heart infusion-sucrose medium, that is, sucrose with a final concentration of 1% is added to the brain heart infusion medium. Sucrose needs to be made into 20% stock solution in advance and sterilized by filtering with a 0.22 μm sterile filter.
[0051] (3) Streptococcus mutans type strain UA159 and Streptococcus mutans strain UA246 isolated from the oral cavity of de...
Embodiment 3
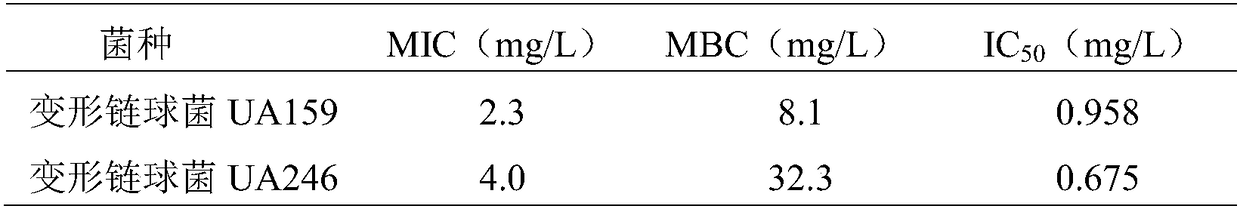
[0054] Example 3: N-(5-bromo-2-methoxyphenyl)-3-methylbenzo[4,5]imidazo[2,1-b]thiazole-2-carboxamide compound on deformed chain Viability detection of cocci planktonic cells
[0055] (1) According to the method described in Example 1, N-(5-bromo-2-methoxyphenyl)-3-methylbenzo[4,5]imidazo[2,1-b]thiazole- 2-formamide, accurately weigh the compound with an analytical balance, and add DMSO to dissolve it, then filter and sterilize it with a sterile filter with a pore size of 0.22 μm, and make a stock solution with a final concentration of 1024 mg / L, and store it in - 20°C for use;
[0056] Prepare Streptococcus mutans UA159 and UA246 bacterium liquid according to the method described in embodiment 2, it is cultivated to logarithmic phase and makes Streptococcus mutans bacterium liquid OD 600 nm=0.8~1.0, diluted with brain heart infusion medium to a final concentration of 5×10 5 cfu / ml for use.
[0057] (2) Detection of N-(5-bromo-2-methoxyphenyl)-3-methylbenzo[4,5]imidazo[2,1-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com