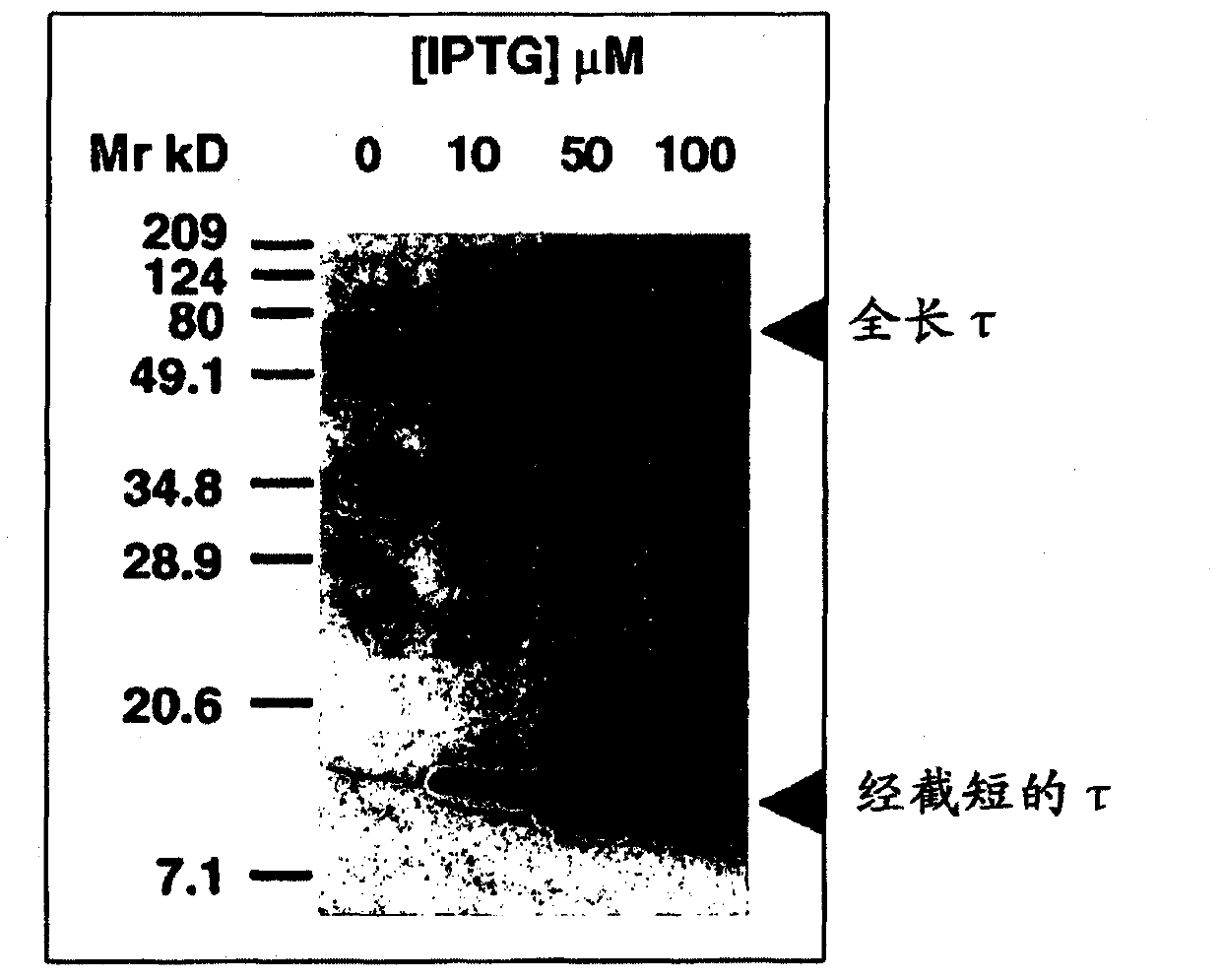
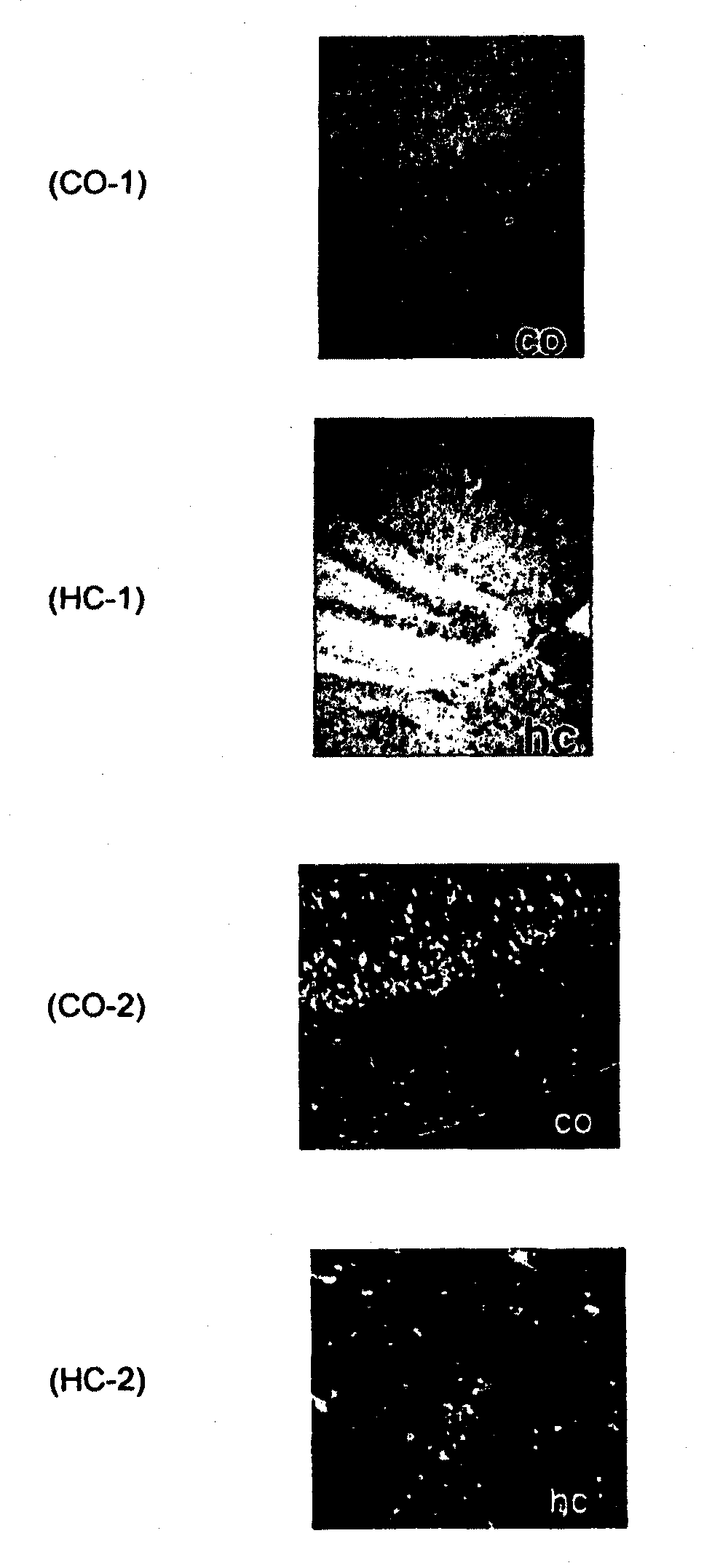
Ligands for aggregated tau molecules
A helical, self-contained technique in the field of ligands for neuropathic staging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0828] In one embodiment, the compound is selected from compounds of the following formulae and pharmaceutically acceptable salts, hydrates and solvates thereof.
[0829] Compounds, wherein -Q- is -NHC(O)-; -NR 1 C(O)-; -C(O)NH-; or -C(O)NR 1 -.
[0830] Benzothiazole compounds
[0831] Non-fluorinated methoxy-amides
[0832]
[0833]
[0834]
[0835] In one embodiment, the compounds are independently selected from:
[0836] ABMA-04; ABMA-05; ABMA-06; ABMA-07; ABMA-08; ABMA-09; ABMA-10; ABMA-11; ABMA-13; ABMA-14; ABMA-15; and ABMA-16.
[0837] In one embodiment, the compounds are independently selected from:
[0838] ABMA-04; ABMA-05; ABMA-06; ABMA-07; ABMA-08; ABMA-09; ABMA-10; ABMA-11; and ABMA-13.
[0839] Fluorinated methoxy-amide
[0840]
[0841]
[0842]
[0843] In one embodiment, the compounds are independently selected from:
[0844] ABFMA-04; ABFMA-05; ABFMA-06; ABFMA-07; ABFMA-08; ABFMA-09; ABFMA-11; ABFMA-12; ABFMA-14; ABFMA-15;
[08...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com