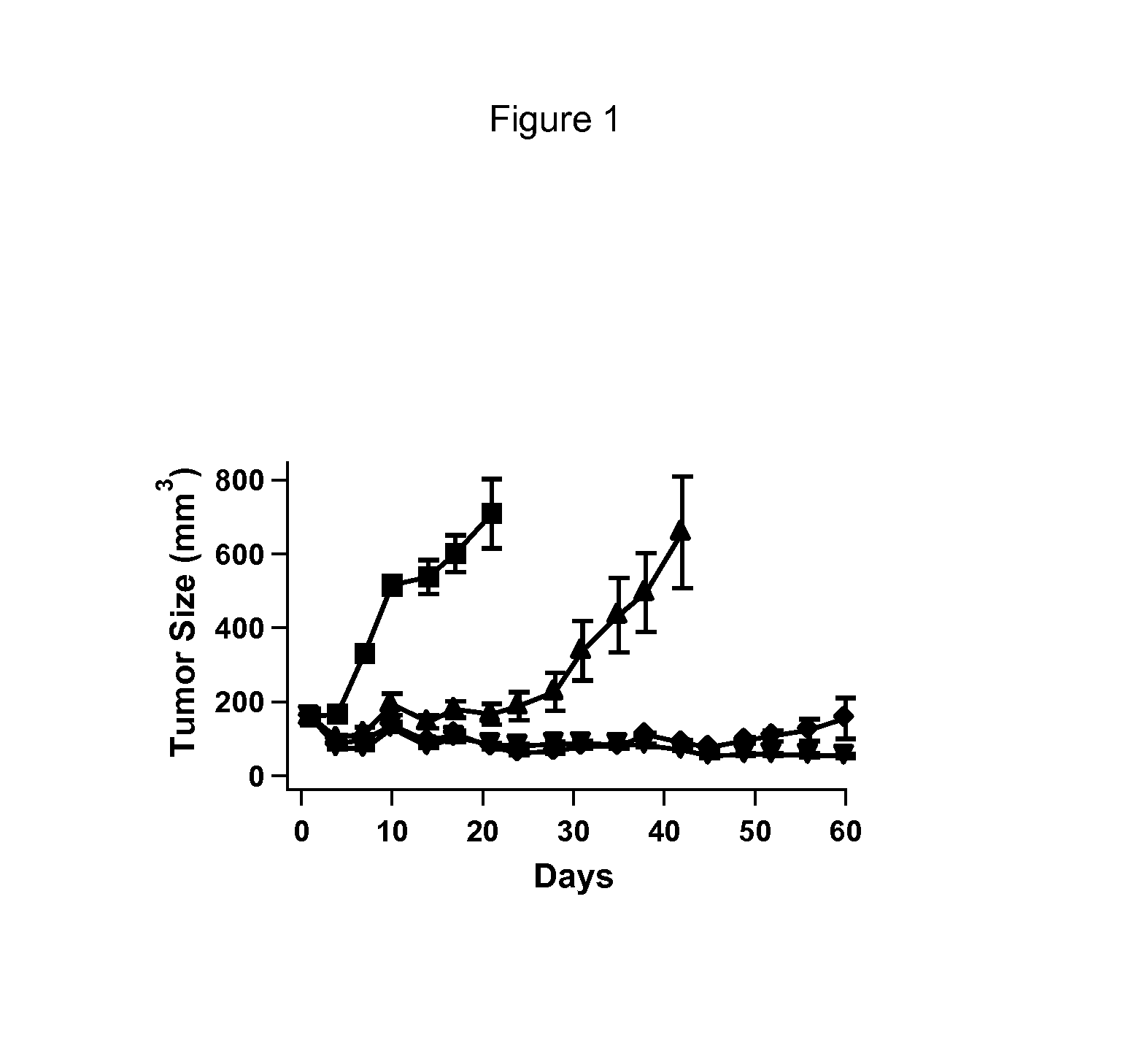
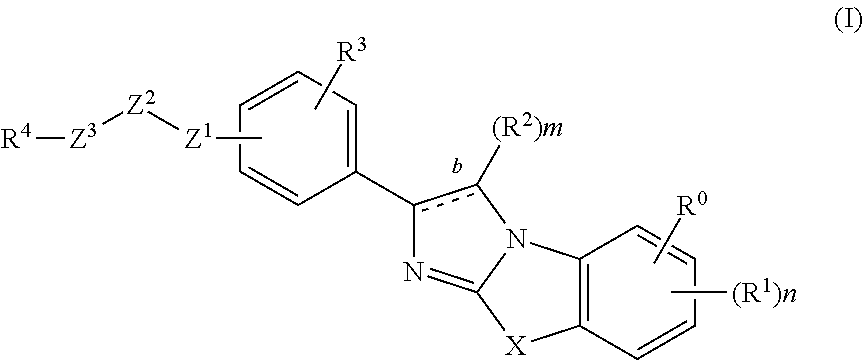
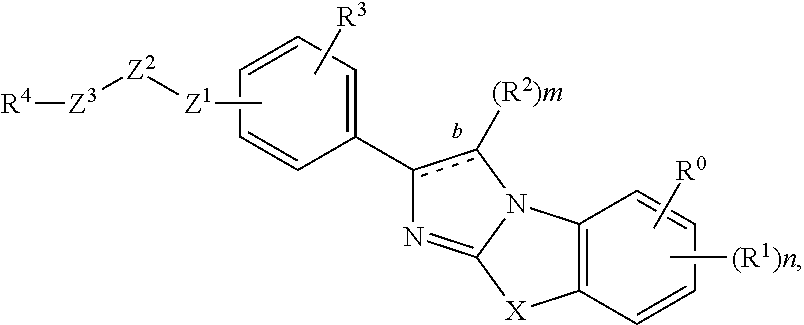
Imidazolothiazole compounds for the treatment of disease
a technology of imidazolothiazole and compounds, which is applied in the field ofimidazolothiazole compounds for the treatment of diseases, can solve the problems of impaired rtks deactivation and virus life cycle disruption, and achieve the effect of effectively treating, managing or ameliorating the disease or ameliorating or eliminating one or more symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-(5-tert-Butyl-isoxazol-3-yl)-N′-imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea [Compound A1]
[0469]A. To prepare the intermediate 2-(4-nitrophenyl)imidazo[2,1-b][1,3]benzothiazole, 2-aminobenzothiazole (751 mg, 5 mmol) and 2-bromo-4′-nitroacetophenone (1.22 g, 5 mmol) were dissolved in ethanol and heated to reflux overnight. The solution was then cooled at room temperature for 24 hours. The precipitate was collected by filtration, washed with methanol and dried under vacuum.
[0470]B. To prepare the 2-(4-amino-phenyl)imidazo[2,1-b][1,3]benzothiazole, the intermediate from step A (428 mg, 1.5 mmol) was prepared as a suspension in isopropyl alcohol, and to it was added iron powder (419 mg, 7.5 mmol). The suspension was heated to reflux overnight with vigorous stirring. Completion of the reaction was confirmed by LCMS. 1N HCl was added to the mixture and allowed to cool to room temperature. The precipitate was collected by filtration and washed with several volumes of m...
example 2
Preparation of 1-(5-tert-Butyl-isoxazol-3-yl)-3-[4-(7-morpholin-4-yl-benzo[d]imidazo[2,1-b]thiazol-2-yl)-phenyl]urea; [Compound A6]
[0477]A. Preparation of the intermediate 6-morpholin-4-yl-benzothiazol-2-amine: To a solution of 4-N-morpholinoaniline (1.78 g, 10 mmol) in acetic acid (20 mL) was added NH4SCN (2.28 g, 30 mmol) in small amounts several times. After stirring the mixture for 30 minutes, a solution of bromine in acetic acid (1.6 g in 5 mL) was added to the mixture and stirred overnight at room temperature. The mixture was then heated at 90° C. for 30 minutes, and then was cooled and neutralized with saturated NaHCO3, and then extracted three times with CH2Cl2. The combined organic phases were dried over MgSO4 and concentrated to dryness. To the residue was added 30 mL of 10% HCl and neutralized with saturated NaHCO3, to give a brown solid (1.541 g, 66%).
[0478]B. Preparation of the intermediate 7-morpholin-4-yl-2-(4-nitro-phenyl)-imidazo[2,1-b][1,3]benzothiazole: A mixture ...
example 3
Preparation of N-(5-tert-Butyl-isoxazol-3-yl)-N′-{4-[7-(2-morpholin-4-yl-ethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl}urea [Compound B1]
[0482]A. The intermediate 2-amino-1,3-benzothiazol-6-ol was prepared according to a slightly modified literature procedure by Lau and Gompf: J. Org. Chem. 1970, 35, 4103-4108. To a stirred solution of thiourea (7.6 g, 0.10 mol) in a mixture of 200 mL ethanol and 9 mL concentrated hydrochloric acid was added a solution of 1,4-benzoquinone (21.6 g, 0.20 mol) in 400 mL of hot ethanol. The reaction was stirred for 24 hours at room temperature and then concentrated to dryness. The residue was triturated with hot acetonitrile and the resulting solid was filtered and dried.
[0483]The free base was obtained by dissolving the hydrochloride salt in water, neutralizing with sodium acetate, and collecting the solid by filtration. The product (2-amino-1,3-benzothiazol-6-ol) was obtained as a dark solid that was pure by LCMS (M+H=167) and NMR. Yield: 13.0 g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com