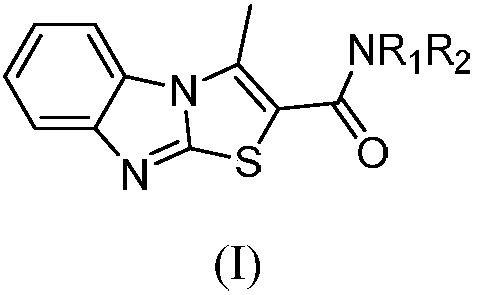
Benzimidazothiazole carboxamide compound and application thereof
A kind of imidazo, formamide technology, applied in the field of medicine, can solve the problem of serious drug resistance and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
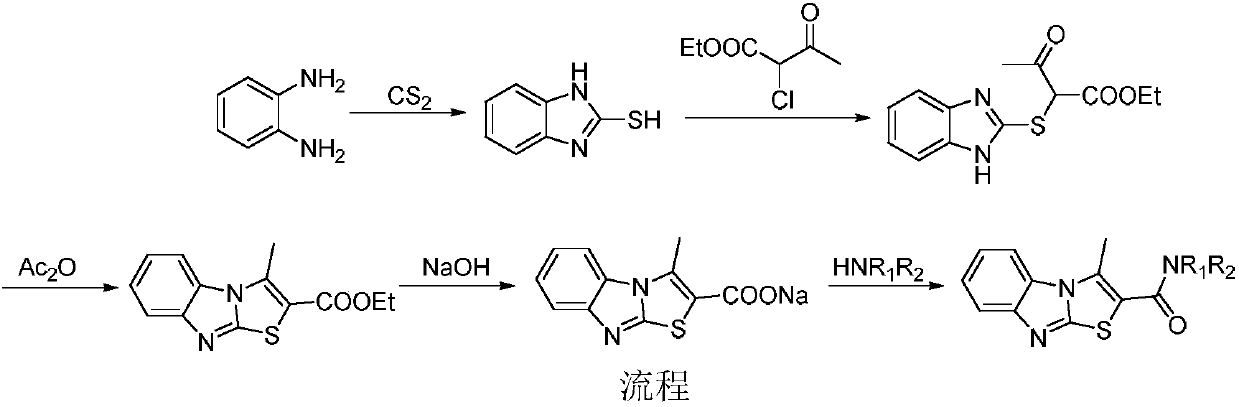
[0022] Example 1: Preparation of N-(2-methylphenyl)-3-methylbenzo[4,5]imidazo[2,1-b]thiazole-2-carboxamide (compound X01)
[0023] Step A: Preparation of 2-Mercaptobenzimidazole
[0024] Place o-phenylenediamine (21.61g, 0.20mol), carbon disulfide (18.24g, 0.24mol), sodium carbonate (14.84g, 0.14mol) and 300mL of water in a 500mL eggplant-shaped bottle, and heat to reflux for 7 hours. Cool to room temperature, suction filter, and dry to obtain 29.4 g of white solid, yield 98.0%, m.p.290-292°C (lit.: 303-305°C), ESI-MS (m / z): 151.1 ([M+H ] + );IR(KBr):υ3441,3153,3114,2979,2877,1618,1513,1467,1215; 1 H-NMR (400MHz, DMSO): δ7.10~7.15 (m, 4H), 12.51 (s, 2H).
[0025] Step B: Preparation of ethyl 2-((1H-benzo[d]imidazol-2-yl)mercapto)-3-oxybutanoate
[0026] Put 2-mercaptobenzimidazole (18.00g, 0.12mol), sodium bicarbonate (30.24g, 0.36mol), ethyl 2-chloroacetoacetate (22.96g, 0.14mol) and 200mL of ethanol in a 1000mL eggplant-shaped flask , after stirring at room temperature ...
Embodiment 2
[0032] Example 2: Preparation of N,3-dimethyl-N-phenylbenzo[4,5]imidazo[2,1-b]thiazole-2-carboxamide (compound X02)
[0033] Referring to the preparation method of Example 1, 0.16 g of a white solid was obtained, with a yield of 49%, m.p.: 167-169° C.; IR: (KBr, cm -1 )3444(s), 2924(s), 2854(s), 1638(s), 1455(m), 1383(s), 741(s), 700(s); 1 HNMR(400MHz,DMSO):δ2.75(s,3H,CH 3 ),3.40(s,3H,CH 3 ),7.22-7.26(m,1H,Ar-H),7.28-7.31(m,1H,Ar-H),7.32-7.36(m,1H,Ar-H),7.38(d,4H,Ar-H , J=4.3Hz), 7.64 (d, 1H, Ar-H, J=8.0Hz), 7.93 (d, 1H, Ar-H, J=8.0Hz); ESI-MS (m / z): 322.0 ( [M+H] + ).
Embodiment 3
[0034] Example 3: Preparation of 3-methyl-N-(3-nitrophenyl)benzo[4,5]imidazo[2,1-b]thiazole-2-carboxamide (compound X03)
[0035] Referring to the preparation method of Example 1, 0.07 g of a white solid was obtained, with a yield of 20%, m.p.: 258-260° C.; IR: (KBr, cm -1 )3441(s), 2924(s), 2853(s), 1637(s), 1456(m), 1384(s), 736(s), 620(s); 1 HNMR(400MHz,DMSO):δ3.09(s,3H,CH 3 ),7.32-7.36(m,1H,Ar-H),7.41-7.45(m,1H,Ar-H),7.69(t,1H,Ar-H,J=8.2Hz),7.75(d,1H, Ar-H, J=8.1Hz), 8.01(dd, 1H, Ar-H, J=8.1, 1.8Hz), 8.11(dd, 2H, Ar-H, J=8.1, 2.4Hz), 8.68(t, 1H, Ar-H, J=2.1Hz), 10.74 (s, 1H, NH); ESI-MS (m / z): 350.7 ([M-H] - ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com