Preparation method of dexmedetomidine
A dexmedetomidine and group technology, which is applied in the field of organic chemical synthesis, can solve the problems of poor atom economy, high three-waste emission and high cost, and achieves the effects of reducing environmental protection pressure, avoiding the generation of process impurities, and reducing pressure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
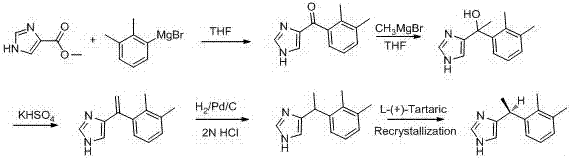
Embodiment 1
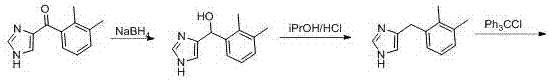
[0035] step 1:
[0036] (2,3-dDimethylphenyl)(1H-imidazol-4-yl)methanol
[0037]
[0038] Add 60 g of (2,3-dimethylphenyl)(1H-imidazol-4-yl)methanone into a 1000 mL four-neck flask, add 300 mL of methanol, and add 34.53 g sodium borohydride, temperature control below 0 ℃, there is gas generation and exotherm, after adding sodium borohydride for 20 minutes, sampling TLC shows that the reaction is complete, carry out post-treatment: control temperature below 10 ℃, add 100 mL ice water to quench, Add another 1200 mL of ice water, stir in an ice ethanol bath (0 °C) for 30 min to precipitate a light yellow solid, filter with suction, discard the filtrate, add 200 mL of water to rinse the filter cake, filter with suction, discard the filtrate, and add the filter cake to vacuum Put it in a vacuum drying oven in a drying oven, drying conditions: temperature 60 °C, vacuum -0.09 MPa, time 10 h, and a yellow solid was obtained after drying.
[0039] Step 2:
[0040]
[0041] Tak...
Embodiment 2
[0054] Step 4:
[0055]
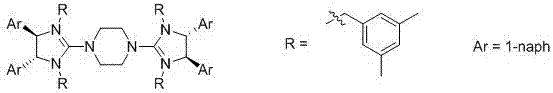
[0056] The residue obtained in the previous step was dissolved in 2000 ml of toluene, and then 2 g of a phase transfer catalyst (structure shown below) was added. Then 20 g of 1,5,7-triazidebicyclo(4.4.0)dec-5-ene were added. Subsequently, 15 g of iodomethane was added and stirred at room temperature for 12 hours. After the reaction was completed, 1000 ml of water was added for liquid separation. The organic phase was desolvated and recrystallized from 700 ml of ethyl acetate. The optical purity of the obtained product was 99.7%.
[0057] The phase transfer catalyst structure is:
[0058]
Embodiment 3
[0060] Step 4:
[0061]
[0062] The residue obtained in the previous step was dissolved in 2000 ml of toluene, and then 3 g of a phase transfer catalyst (structure shown below) was added. Then 20 g of 1,5,7-triazidebicyclo(4.4.0)dec-5-ene were added. Subsequently, 15 g of iodomethane was added and stirred at room temperature for 12 hours. After the reaction was completed, 1000 ml of water was added for liquid separation. The organic phase was desolvated and recrystallized from 700 ml of ethyl acetate. The optical purity of the obtained product was 99.0%.
[0063] The phase transfer catalyst structure is:
[0064]
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com