Preparation method for medetomidine intermediate
A technology of medetomidine and intermediates, applied in the field of medicine, to achieve the effect of low price, simple operation and less waste water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
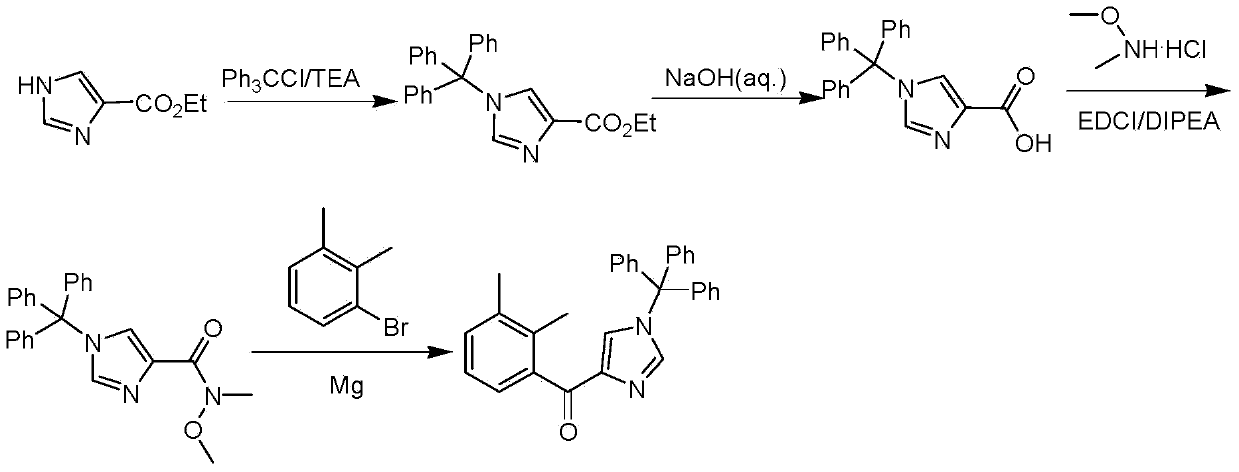
[0025] In a 2L four-necked flask with a mechanical stirrer and a thermometer, add 100g (0.714mol, 1.0eq) ethyl imidazole-4-carboxylate and 1.4L dichloromethane as a solvent, stir to form a suspension, then add 219g Triphenylchloromethane (0.787mol, 1.1eq) and 79.4g (0.787mol, 1.1eq) of triethylamine were slowly heated to between 25°C and 30°C, the reaction liquid became clear, and the reaction was continued for 20h to complete the reaction. Add 200mL of water, stir for 30min, let stand to separate layers, separate the lower organic phase, then extract the aqueous phase with 100mL of dichloromethane, combine the organic phase, wash the organic phase with 200mL of water once, and concentrate the organic phase under reduced pressure to light yellow oily liquid , and then add 500mL ether, a large amount of white solids are produced under stirring, suction filtration, drying to obtain 269g of ethyl 1-trityl-1H-imidazole-4-carboxylate, yield 98%, purity 90% (HPLC).
[0026] Add 600m...
Embodiment 2
[0030] Add 100g (0.714mol, 1.0eq) ethyl imidazole-4-carboxylate and 1.4L dichloromethane as a solvent to a 2L four-neck flask with a mechanical stirrer and a thermometer, stir to form a suspension, add 259g tris Phenylchloromethane (0.93mol, 1.3eq) and 93.8g (0.93mol, 1.3eq) of triethylamine were slowly heated to between 25°C and 30°C, the reaction solution became clear, and the reaction was continued for 20 hours to complete the reaction. Add 200mL of water, stir for 30min, let stand to separate layers, separate the lower organic phase, then extract the aqueous phase with 100mL of dichloromethane, combine the organic phase, wash the organic phase with 200mL of water once, and concentrate the organic phase under reduced pressure to light yellow oily liquid , and then add 500mL ether, a large amount of white solids are produced under stirring, suction filtration, drying to obtain 265g of ethyl 1-trityl-1H-imidazole-4-carboxylate, yield 97%, purity 92% (HPLC).
[0031]Add 600mL ...
Embodiment 3
[0035] Add 100g (0.714mol, 1.0eq) ethyl imidazole-4-carboxylate and 1.4L dichloromethane as a solvent to a 2L four-neck flask with a mechanical stirrer and a thermometer, stir to form a suspension, add 259g tris Phenylchloromethane (0.93mol, 1.3eq) and 93.8g (0.93mol, 1.3eq) of triethylamine were slowly heated to between 25°C and 30°C, the reaction solution became clear, and the reaction was continued for 20 hours to complete the reaction. Add 200mL of water, stir for 30min, let stand to separate layers, separate the lower organic phase, then extract the aqueous phase with 100mL of dichloromethane, combine the organic phase, wash the organic phase with 200mL of water once, and concentrate the organic phase under reduced pressure to light yellow oily liquid , and then add 500mL ether, a large amount of white solids are produced under stirring, suction filtration, drying to obtain 265g of ethyl 1-trityl-1H-imidazole-4-carboxylate, yield 97%, purity 92% (HPLC).
[0036] Add 600mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com