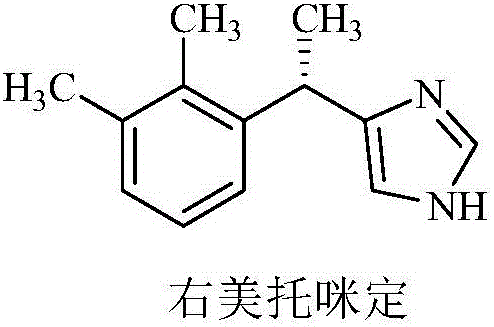
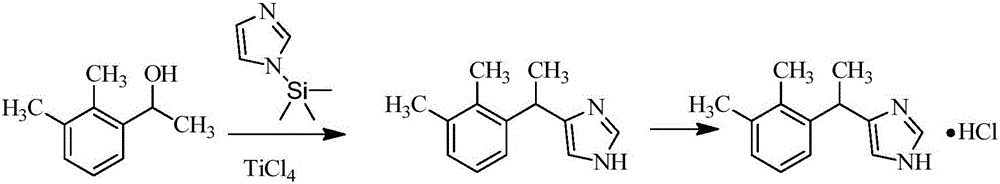
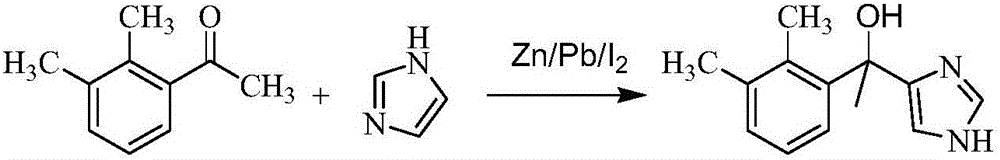
Method for preparing dexmedetomidine and intermediate thereof
A compound and selected technology, applied in organic chemical methods, bulk chemical production, organic chemistry, etc., can solve problems such as multi-industrial impurities, synthetic process inconsistencies, unfavorable purification process development, etc., to avoid process impurities and ensure quality , the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Preparation of 1-(2,3-dimethylphenyl)-1-(1H-imidazol-4-yl)ethanol
[0045] Refer to "Laboratory Chemicals Purification Handbook (Original Work Fifth Edition)" to process the solvent tetrahydrofuran used in the reaction, and add an appropriate amount of water, and use the AKF-1 moisture meter to measure its moisture content to be 0.13%.
[0046]
[0047] In a 500ml reaction flask, the temperature was lower than 50°C, magnesium (5.5g, 224mmol) was added to tetrahydrofuran (140ml), and iodine (10mg) was stirred. Cool down to room temperature, add dropwise a solution of 2,3-dimethylbromobenzene (40.2g, 217mmol) in tetrahydrofuran (120ml, the water content is 0.13% as measured by AKF-1 moisture meter), and stir the mixture at 20-25°C for 1.5h . Heat to reflux for an additional hour, then cool to room temperature. A solution of 1-(1H-imidazol-4-yl)ethanone (19.8g, 180mmol) in tetrahydrofuran (50ml) was added dropwise at 20°C, and stirring was continued at room...
Embodiment 2
[0051] Example 2: Preparation of 1-(triphenylmethyl)-1-(1H-imidazol-4-yl)ethanone
[0052]
[0053] In a 2000ml reaction flask, put 41.3g (282mol) of imidazole-4-ethanone hydrochloride (2), 380ml of DMF, 114ml of triethylamine, under the protection of nitrogen, add triphenylchloromethane dropwise at an internal temperature below 15°C 91.2g (327mmol) was dissolved in 570ml of N,N-dimethylformamide (DMF), and the solution was added in about 1 hour, and the reaction was continued for 1 hour. Cool to below 5°C, filter and drain. Take it out, stir evenly in 1140ml of water, filter, and wash the filter cake with water (200ml). After drying, 88.4 g of solids were obtained, with a yield of 89.0%. (TLC detection, developer: chloroform: methanol: ammoniacal liquor=50:5:1), MS (m / z), 353.3 (MH + ).
Embodiment 3
[0054] Example 3: Preparation of 4-[(2,3-dimethylphenyl)-1-hydroxyethyl]-1-(triphenylmethyl)imidazole
[0055] Refer to "Laboratory Chemicals Purification Handbook (Original Work Fifth Edition)" to process the solvent tetrahydrofuran used in the reaction, and add an appropriate amount of water, and use the AKF-1 moisture meter to measure its moisture content to be 0.09%.
[0056]
[0057] In a 2000ml reaction flask, a solution of 2,3-dimethylbromobenzene (46g, 248mmol) in tetrahydrofuran (800ml, its moisture content measured by AKF-1 moisture meter is 0.09%) was added dropwise to magnesium (6g, 0.25 mmol), an appropriate amount of iodine in tetrahydrofuran (200ml) solution, after the addition, heated to reflux for 1 hour, then cooled to room temperature. The above-prepared solution was added dropwise to a solution of 1-(triphenylmethyl)imidazolone 4 (70 g, 199 mmol) in THF (800 ml) at 20°C. After the addition was complete, the mixture was warmed to room temperature and sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com