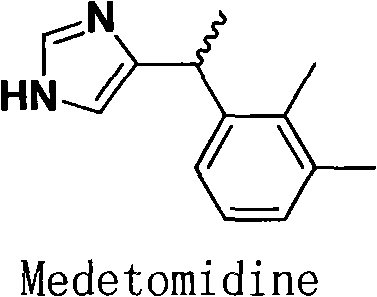
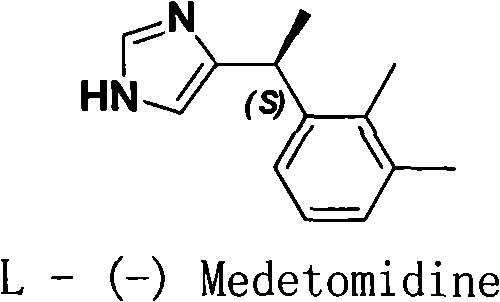
Method for resolution of levorotatory enantiomer and dexiotropic enantiomer of medetomidine
A technology for medetomidine and enantiomers, which is applied in the field of drug synthesis and can solve the problems of complicated operation and difficult to meet the requirements of optical purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation of L-(-)-Medetomidine(base)·S-(+)-BNP
[0016] (±)-4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole 20.0g, S-(+)-phosphoric acid-hydrogen-1,1'-linked-2, Add 17.6g of 2'-naphthyl ester into a 250ml three-necked flask, add 50ml of methanol and stir for 1 hour, add 150ml of anhydrous ether, and place it at 5-8°C for 48 hours to precipitate crystals (if no crystals precipitate during the period, you can add a little levorotatory seed crystal ). After filtration, 15.8 g of (A) was obtained as a white crystalline solid, and the mother liquor (B) was used for later use. A was recrystallized once with anhydrous methanol / ether to obtain L-(-)-Medetomidine (base) S-(+)-BNP 14.7g, mp 181-183℃, [α] D 20 +174.9° (c, 1 g / 100 ml water).
[0017] Preparation of L-(-)-Medetomidine(base)·HCl
[0018] Add 14.7g of L-(-)-Medetomidine (base)·S-(+)-BNP, 120ml of water, and 120ml of ether into a 500ml three-neck flask, add sodium bicarbonate under stirring until the pH value is 1...
Embodiment 2
[0022] D-(+)-Medetomidine (base) R-(-)BNP
[0023] Evaporate the solvent from the mother liquor B obtained after filtering A in Example 1, then add 150ml of anhydrous diethyl ether, place it at 3-8°C for 48 hours, then suck it up, evaporate the filtrate to dryness under normal pressure, dissolve the residue in 110ml of diethyl ether, and wash with 1N hydrochloric acid Extract (70ml×3). The extract was neutralized to strong alkalinity with 20% sodium hydroxide solution, and extracted with ether (75ml×3). The extract was dried with anhydrous sodium sulfate for 5 hours, and then the ether was distilled off. The residue was dissolved in 65 ml of absolute anhydrous methanol, and 11.8 g of R-(-)-phosphate-hydrogen-1,1'-bi-2,2'-naphthyl ester [R-(-)BNP] was added. Stand at 3-8°C for 72 hours to precipitate crystals, filter to obtain white crystalline solid D-(+)-Medetomidine (base)·R-(-)BNP 15.0g, mp 183-184°C, [α] D 20 -173.5°(c, 1g / 100ml water)
[0024] D-(+)-Medetomidine (base...
Embodiment 3
[0029] Preparation of L-(-)-Medetomidine(base)·D-(+)-DBTA
[0030] (±)-4-[1-(2,3-Dimethylphenyl)ethyl]-1H-imidazole 20.0g, D-(+)-dibenzoyl tartaric acid [D-(+)-DBTA ] 17.9g into a 250ml three-neck flask, add 60ml of methanol and stir for 30 minutes, add 150ml of anhydrous ether, and place at 5-10°C for 48 hours to precipitate crystals (if no crystals precipitate during the period, a little L-isomer seed can be added). The obtained white crystalline solid 21.6g (C) was filtered, and the mother liquor (D) was used for later use. C was recrystallized once with absolute ethanol / ether to obtain L-(-)-Medetomidine (base)·(+)-DBTA 18.7g, mp 220-223℃, [α] D 20 +86.6° (c, 1 g / 100 ml water).
[0031] The corresponding hydrochloride L-(-)-Medetomidine (base) HCl 9.3g can be prepared according to the method in Example 1, mp155-157°C, [α] D 20 -52.9° (c, 1 g / 100 ml water). And free base L-(-)-Medetomidine (base) 6.2g. mp148-150°C, [α] D 20 -74.8°(c, 1g / 100ml methanol)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com