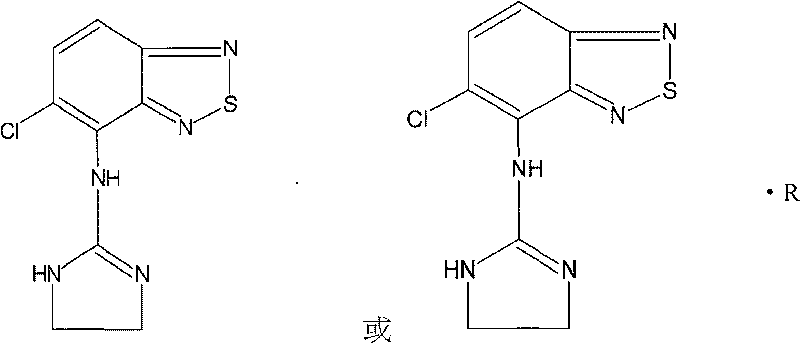
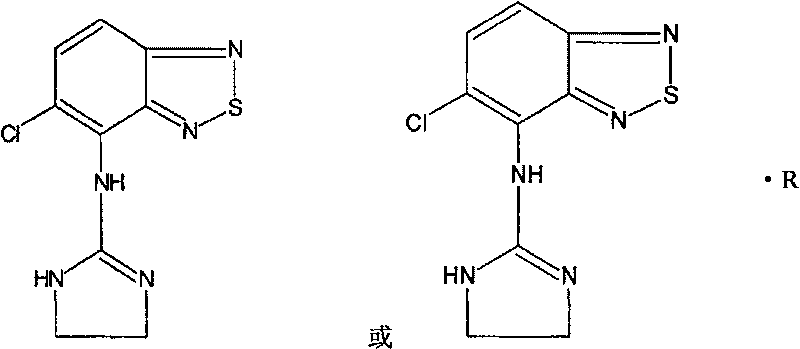
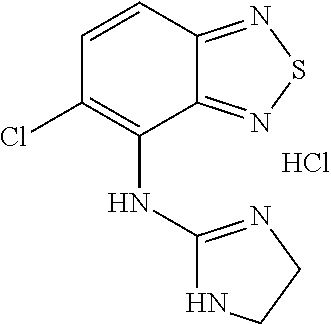
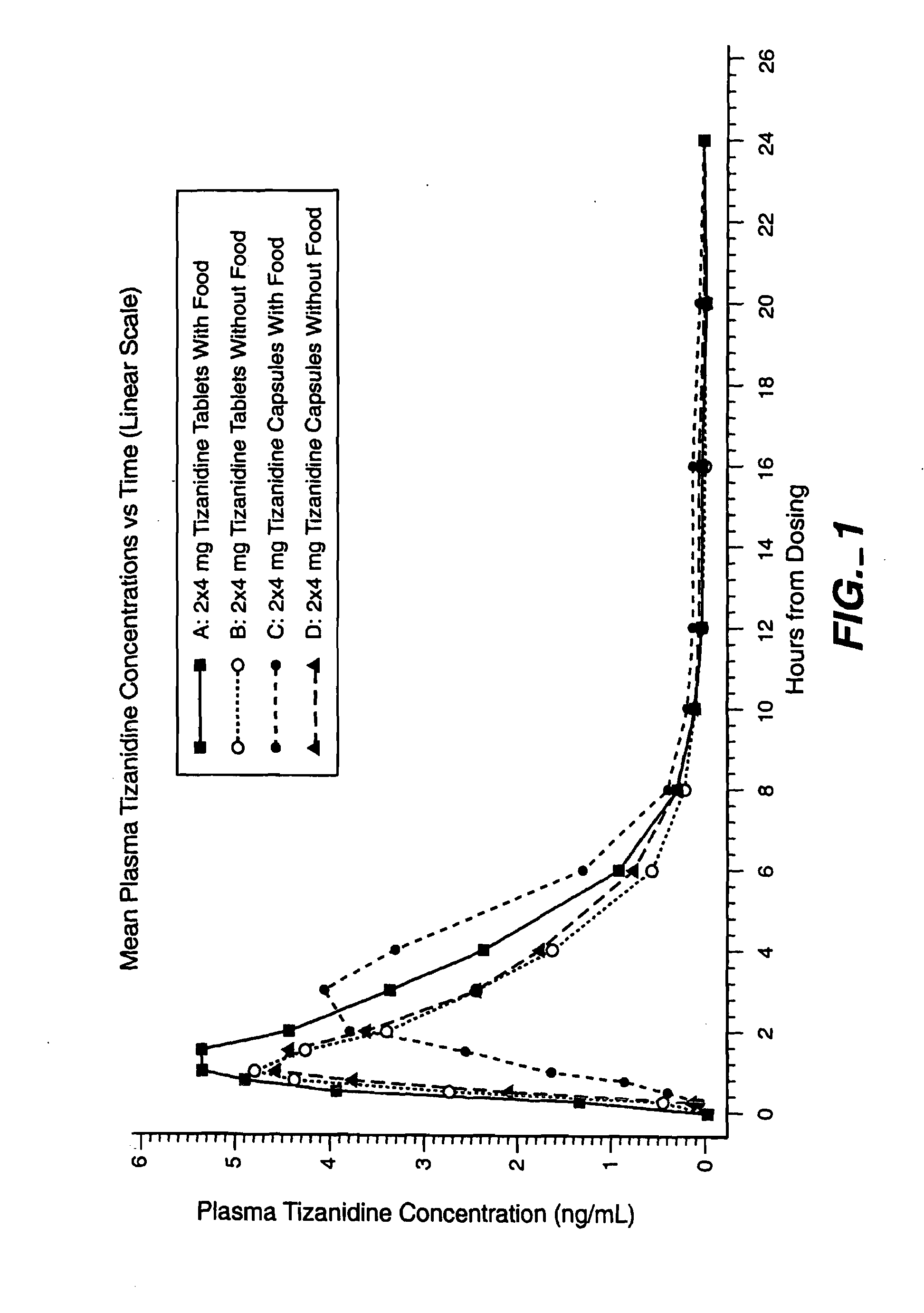
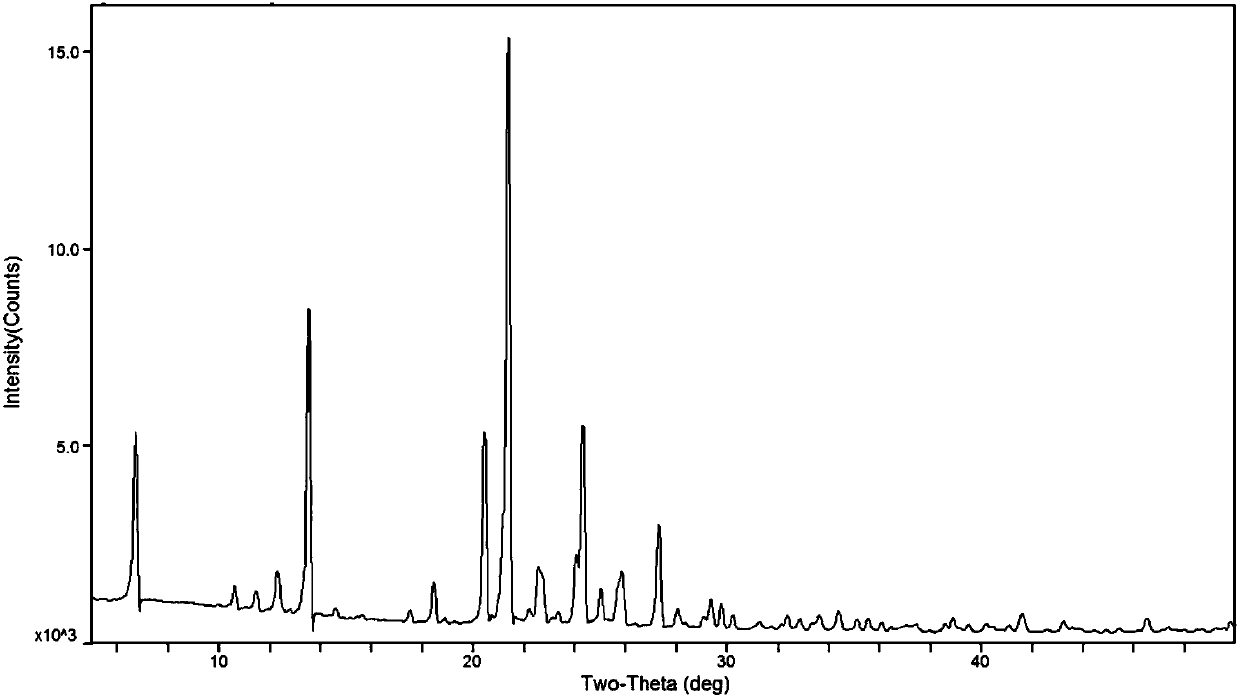
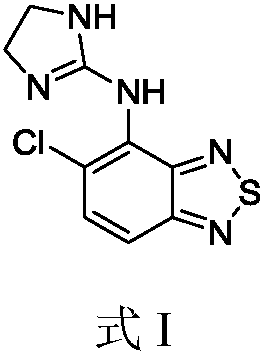
Patents
Literature
31 results about "Tizanidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
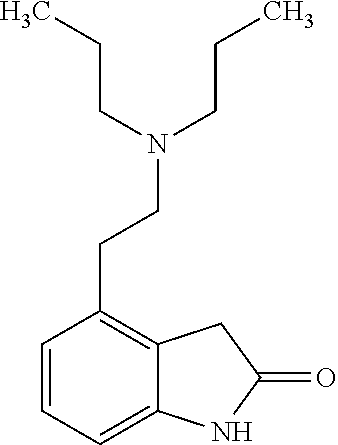
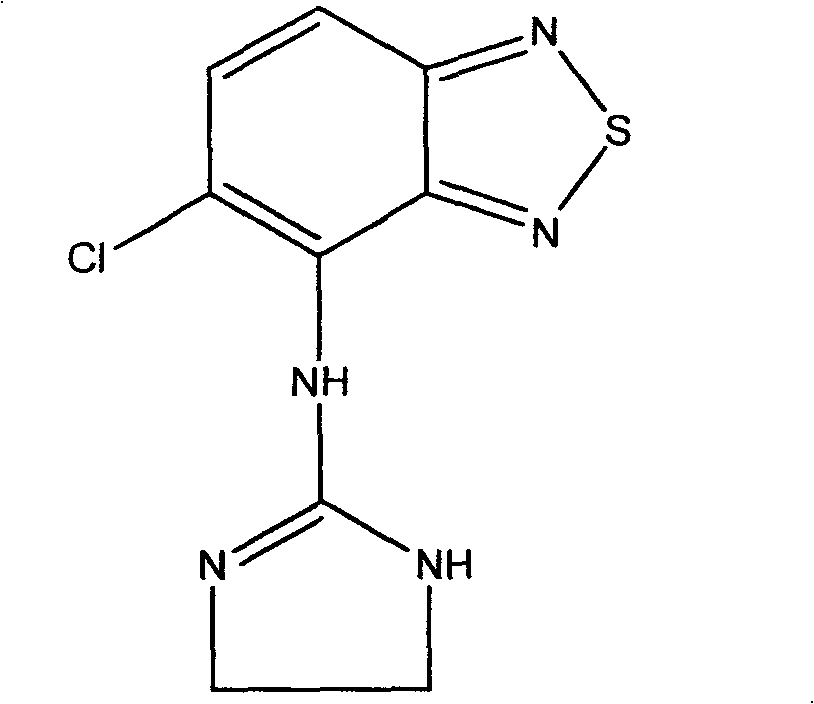
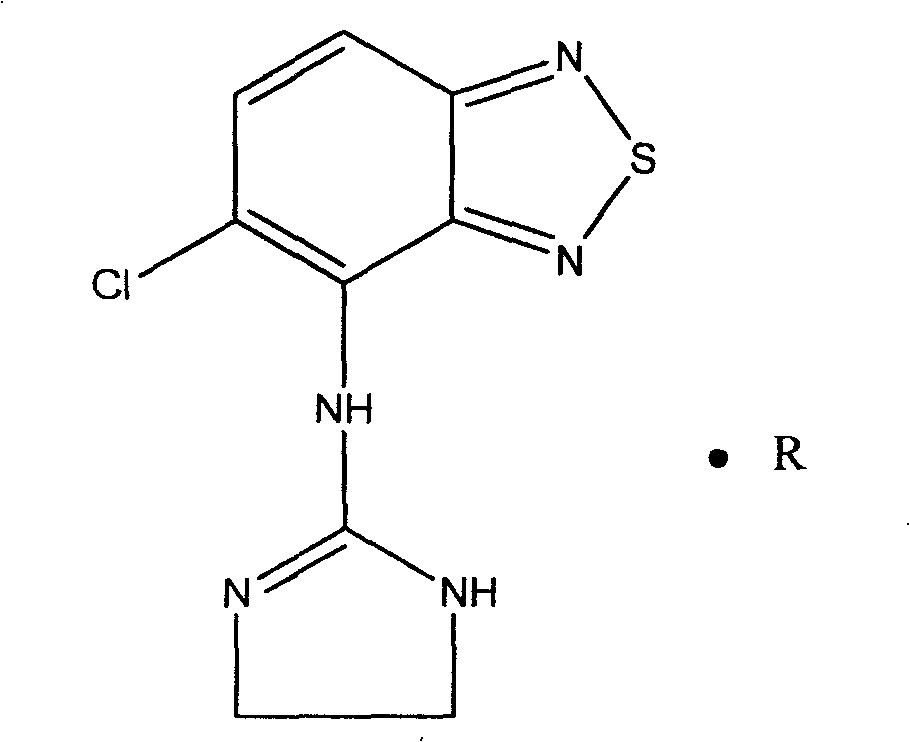
This medication is used to treat muscle spasms caused by certain conditions (such as multiple sclerosis, spinal cord injury).
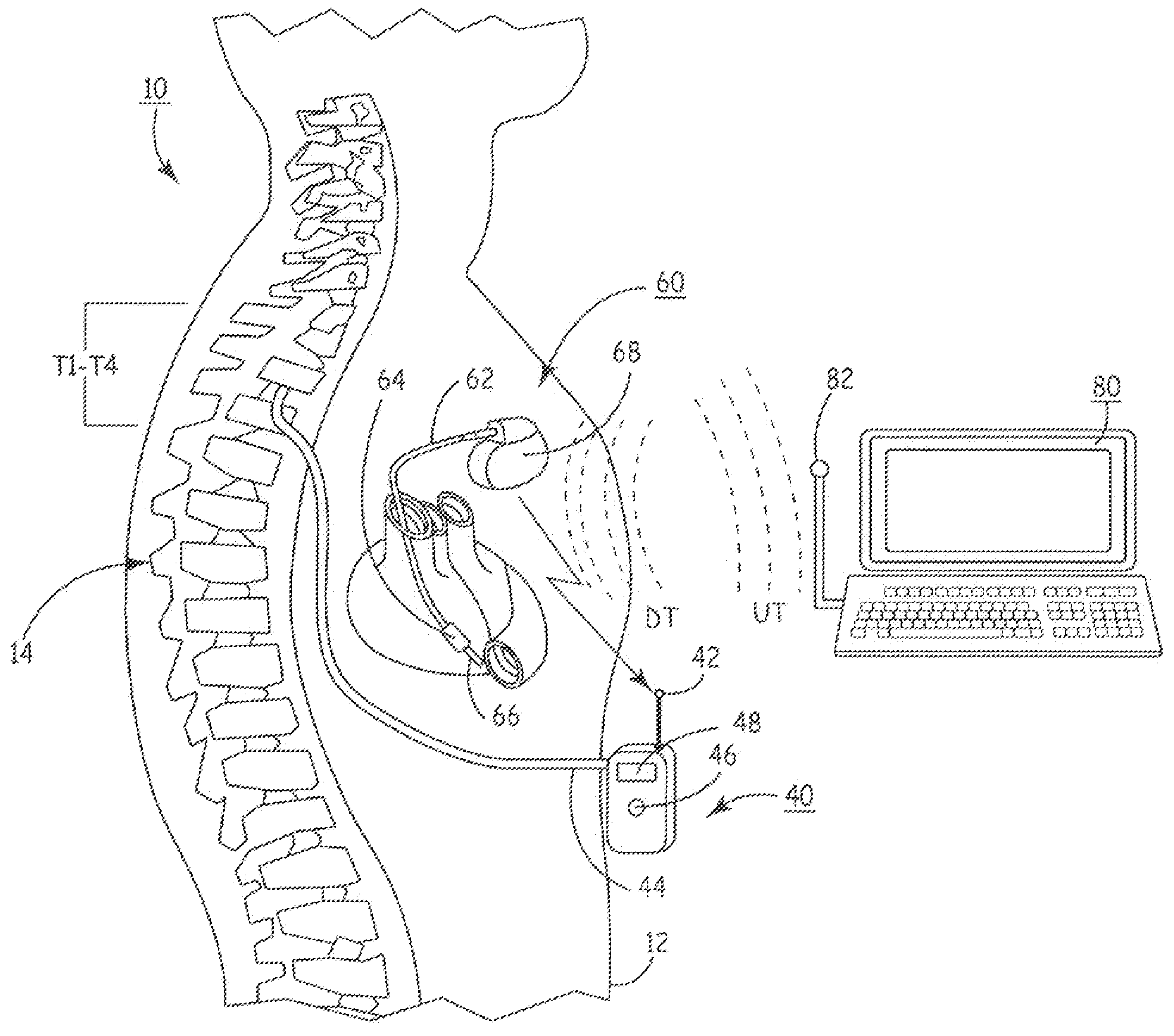
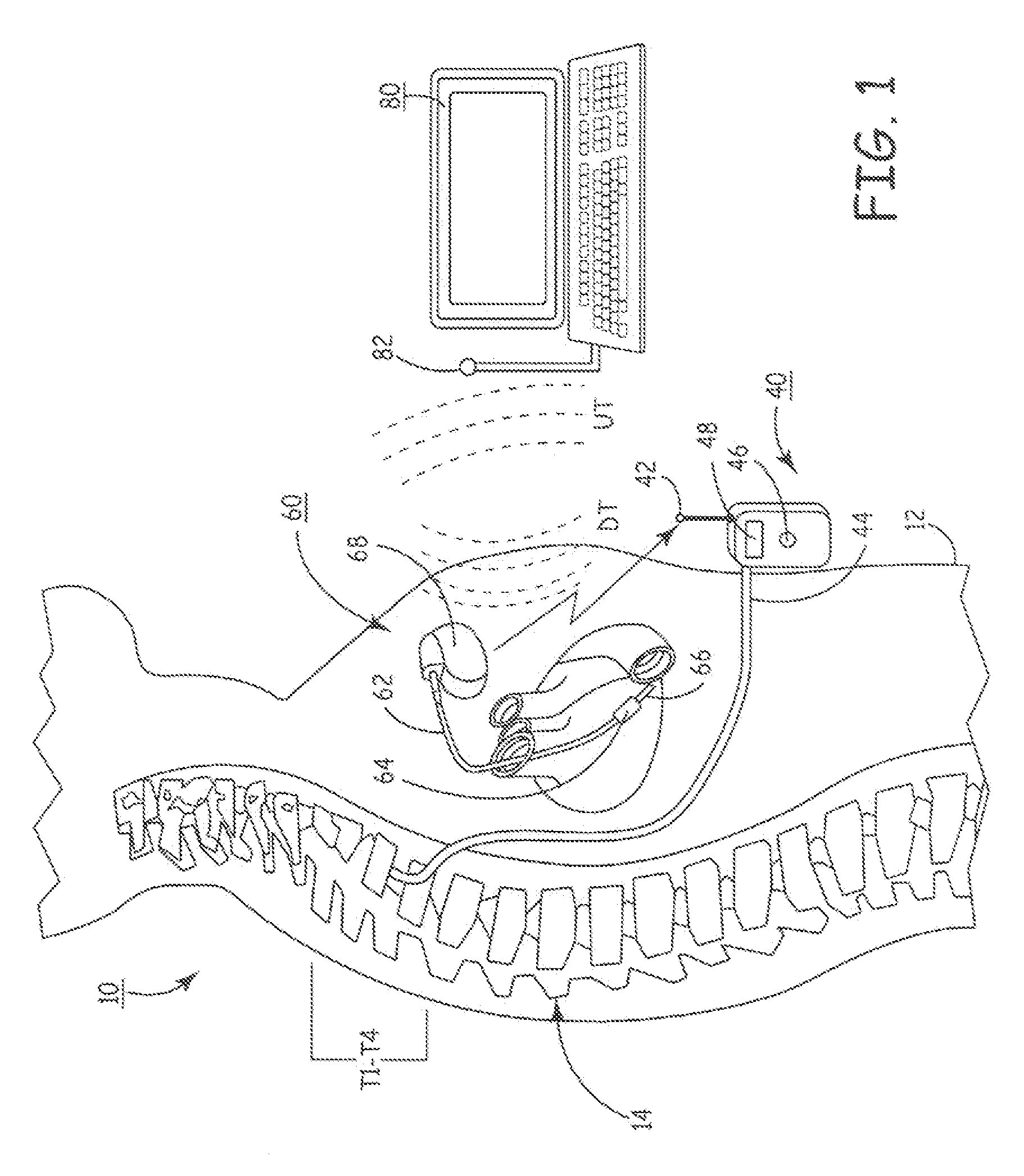
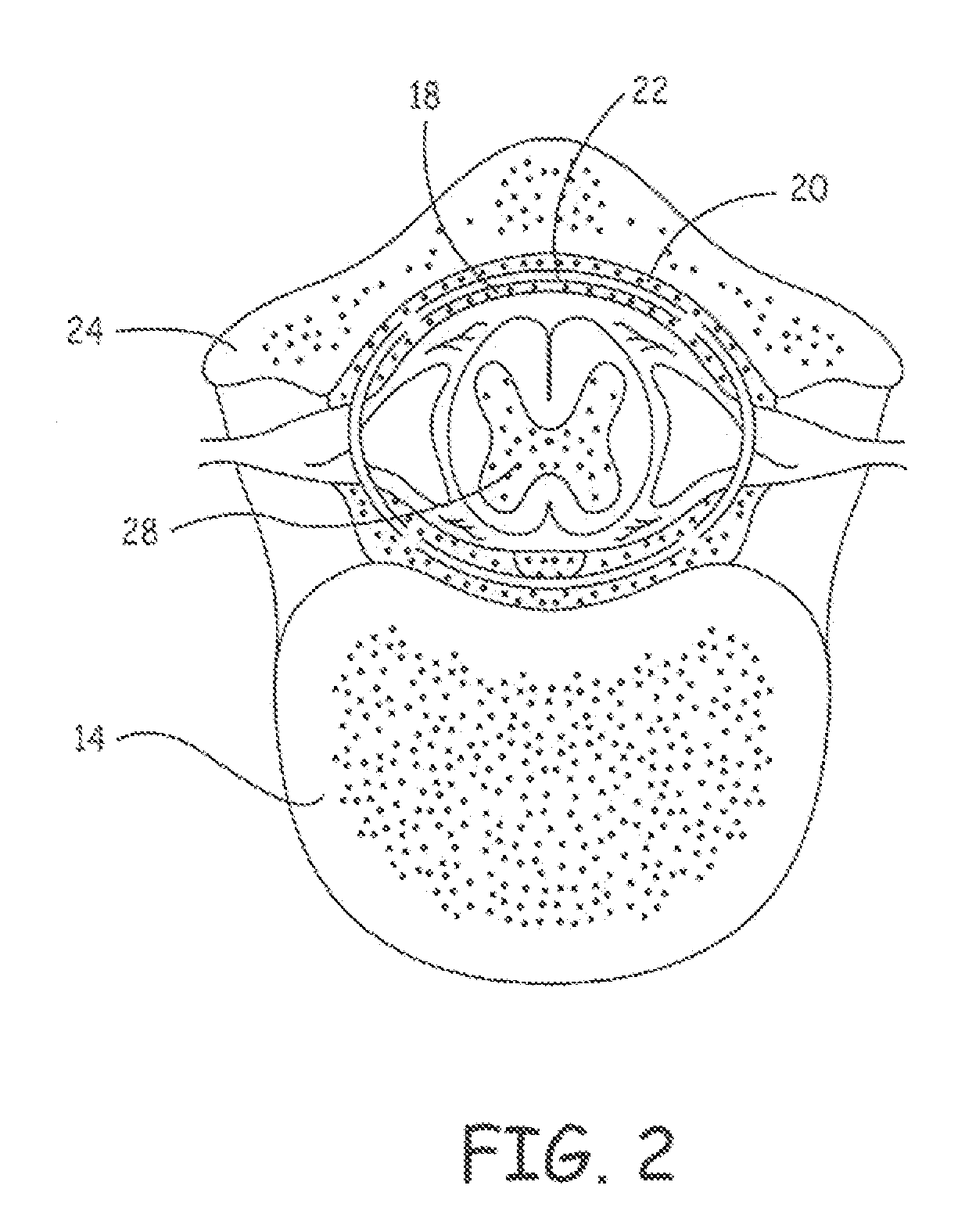
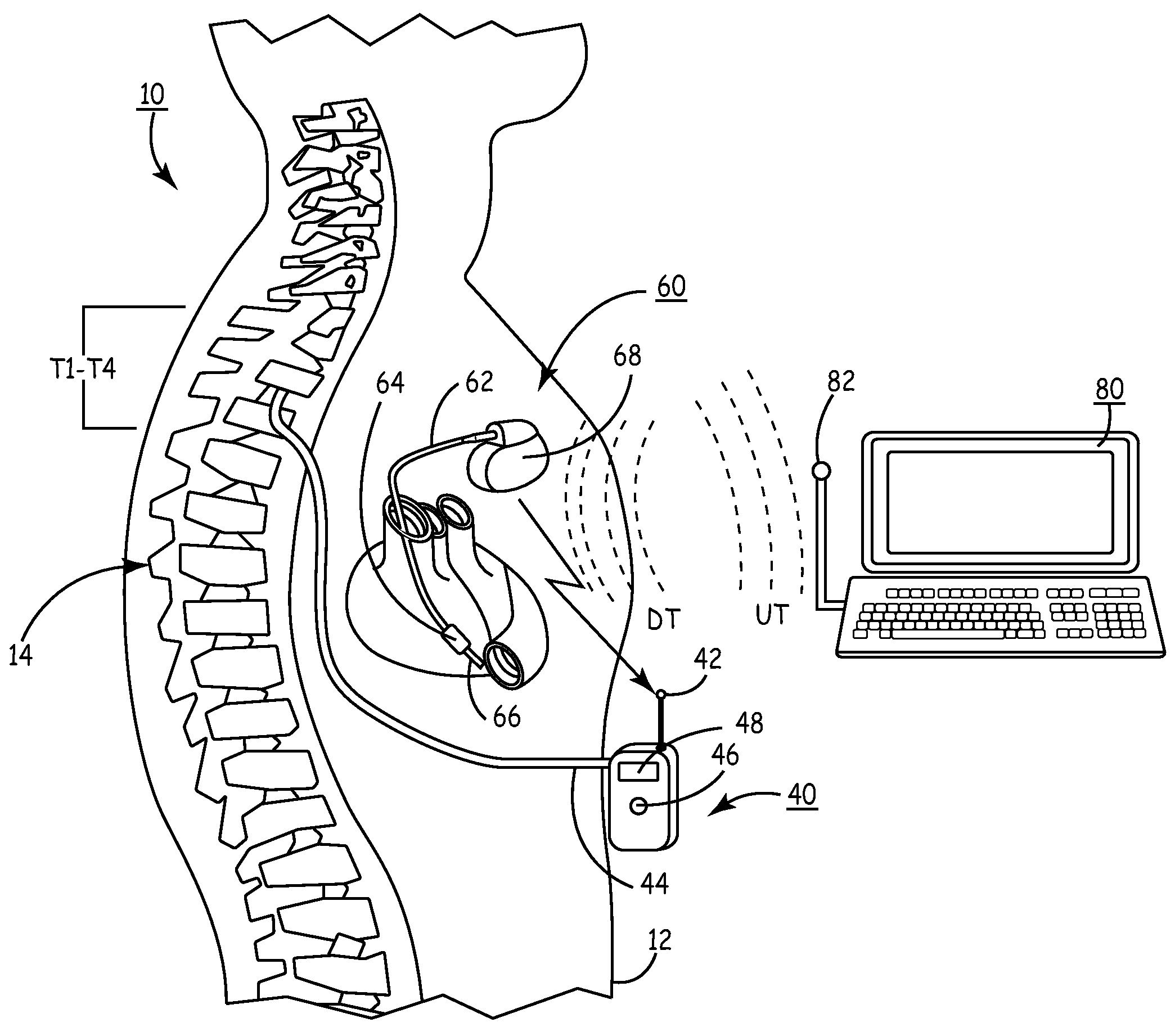
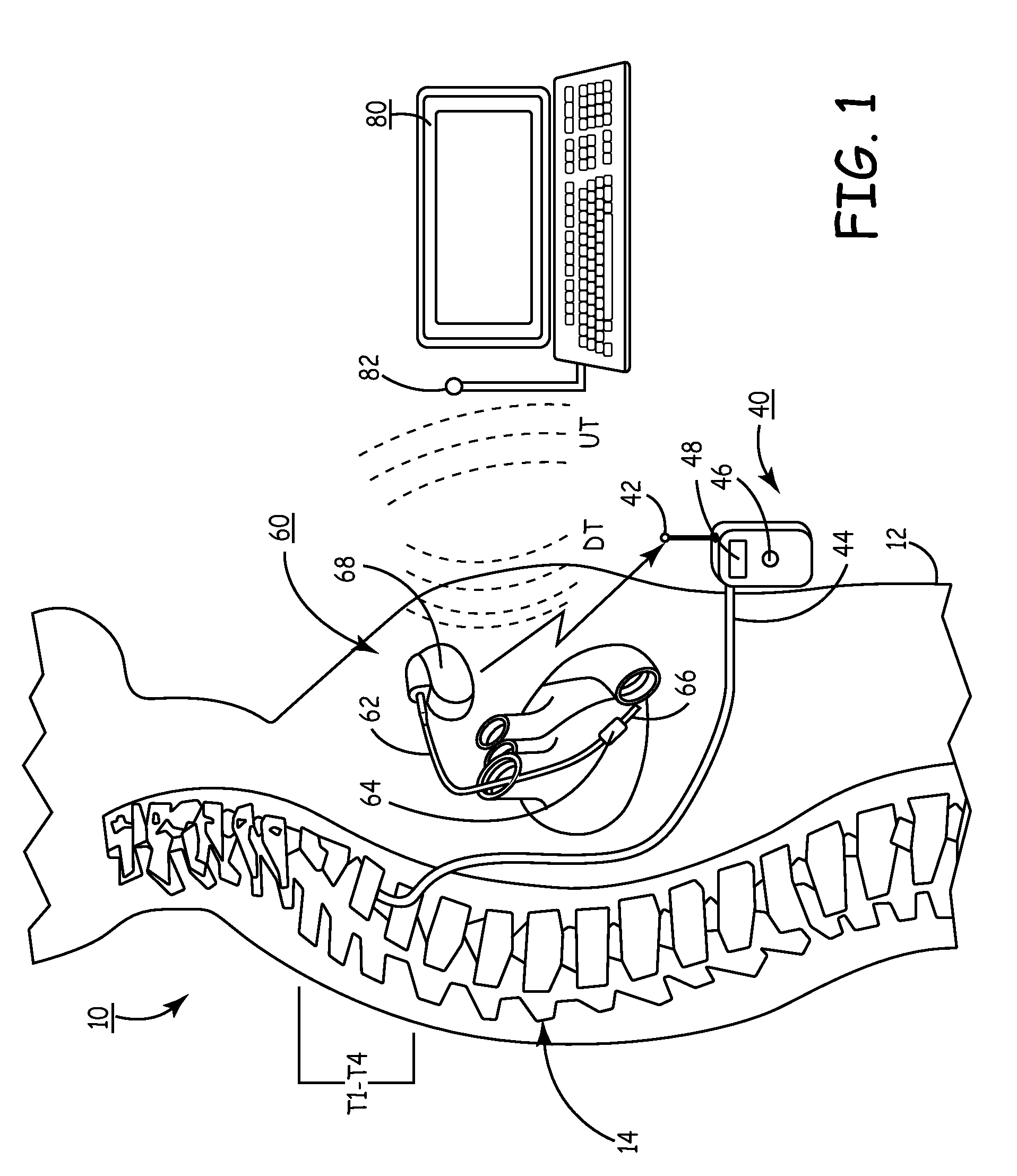
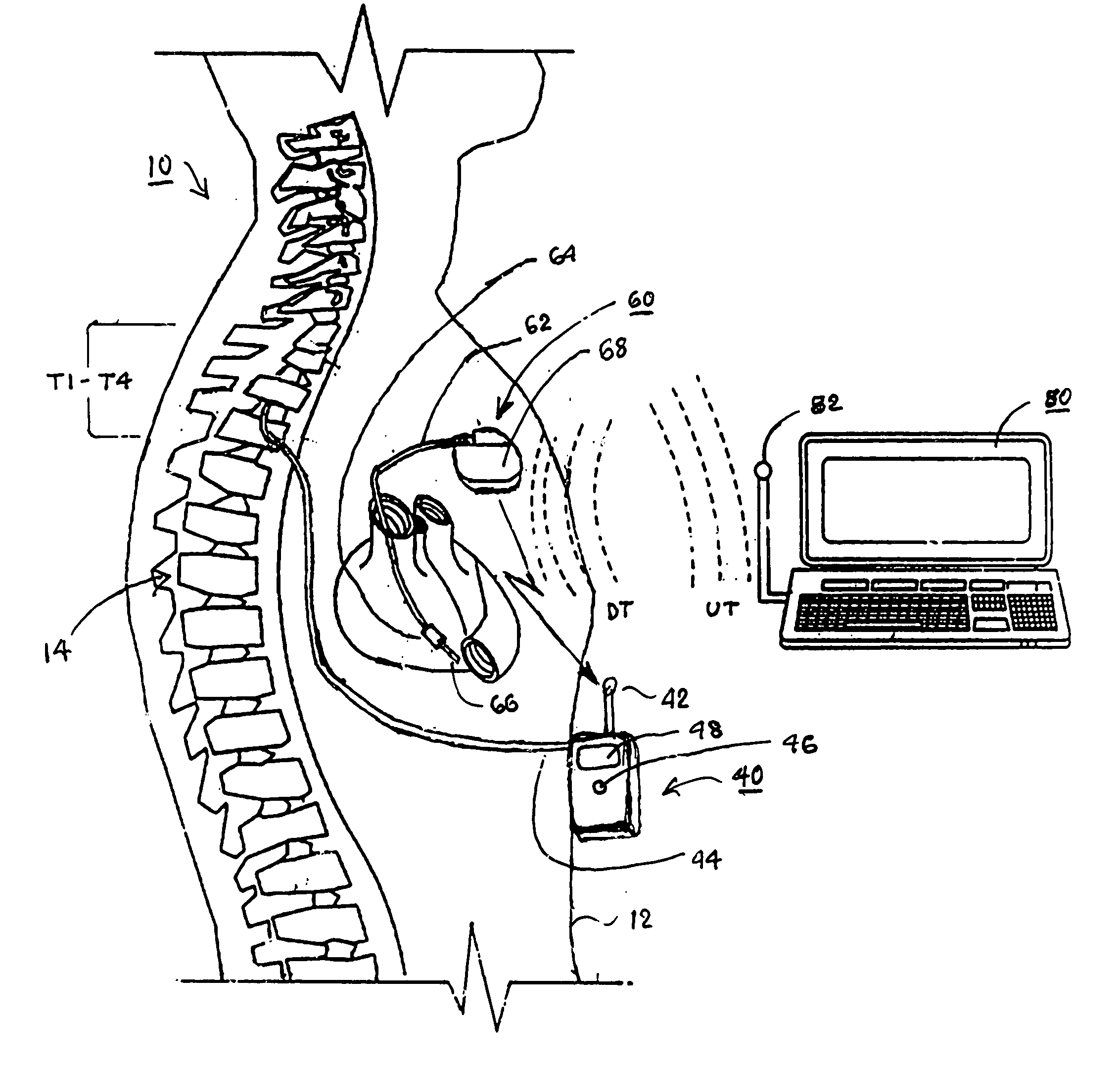
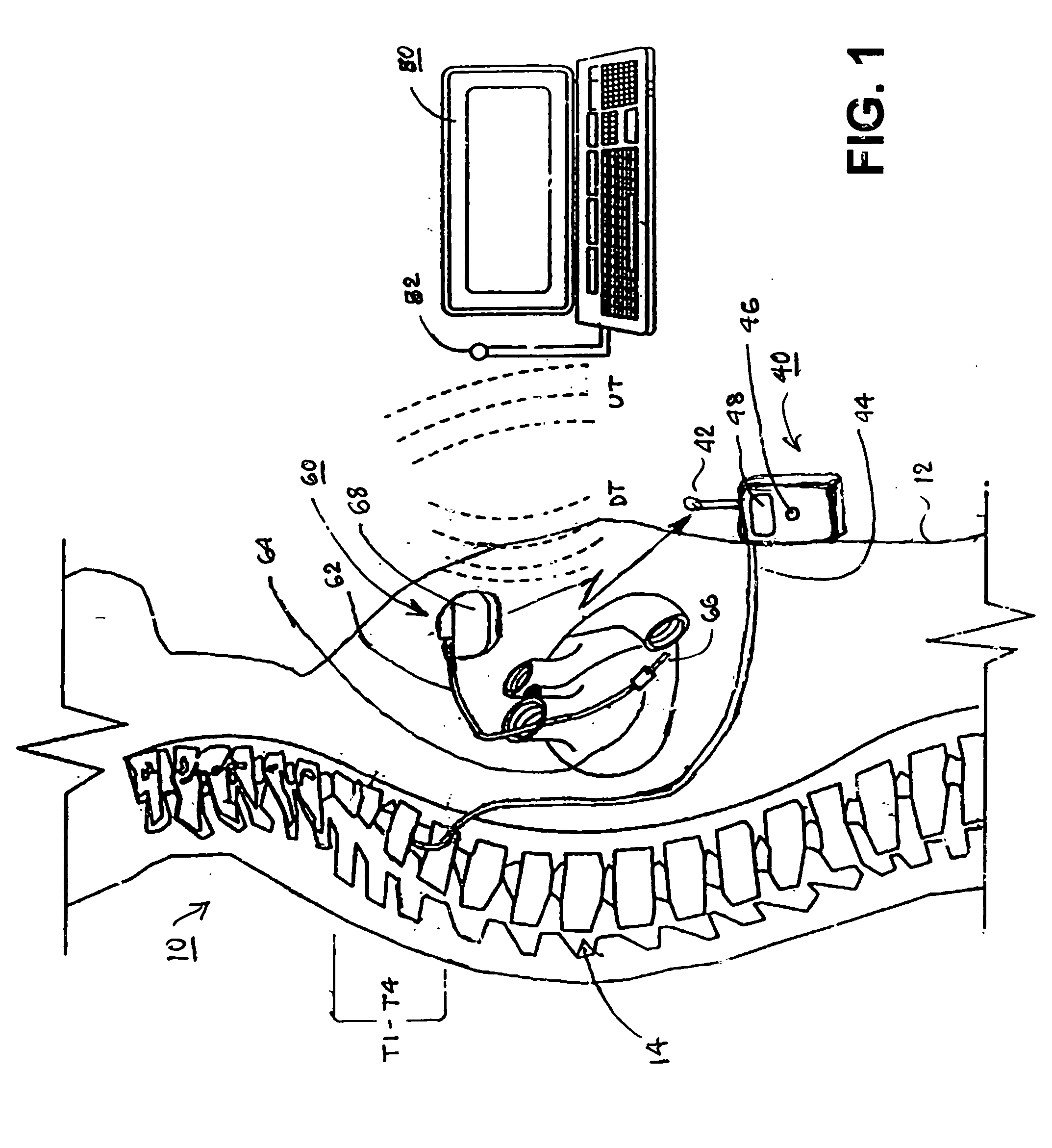
Delivery of a sympatholytic cardiovascular agent to the central nervous system
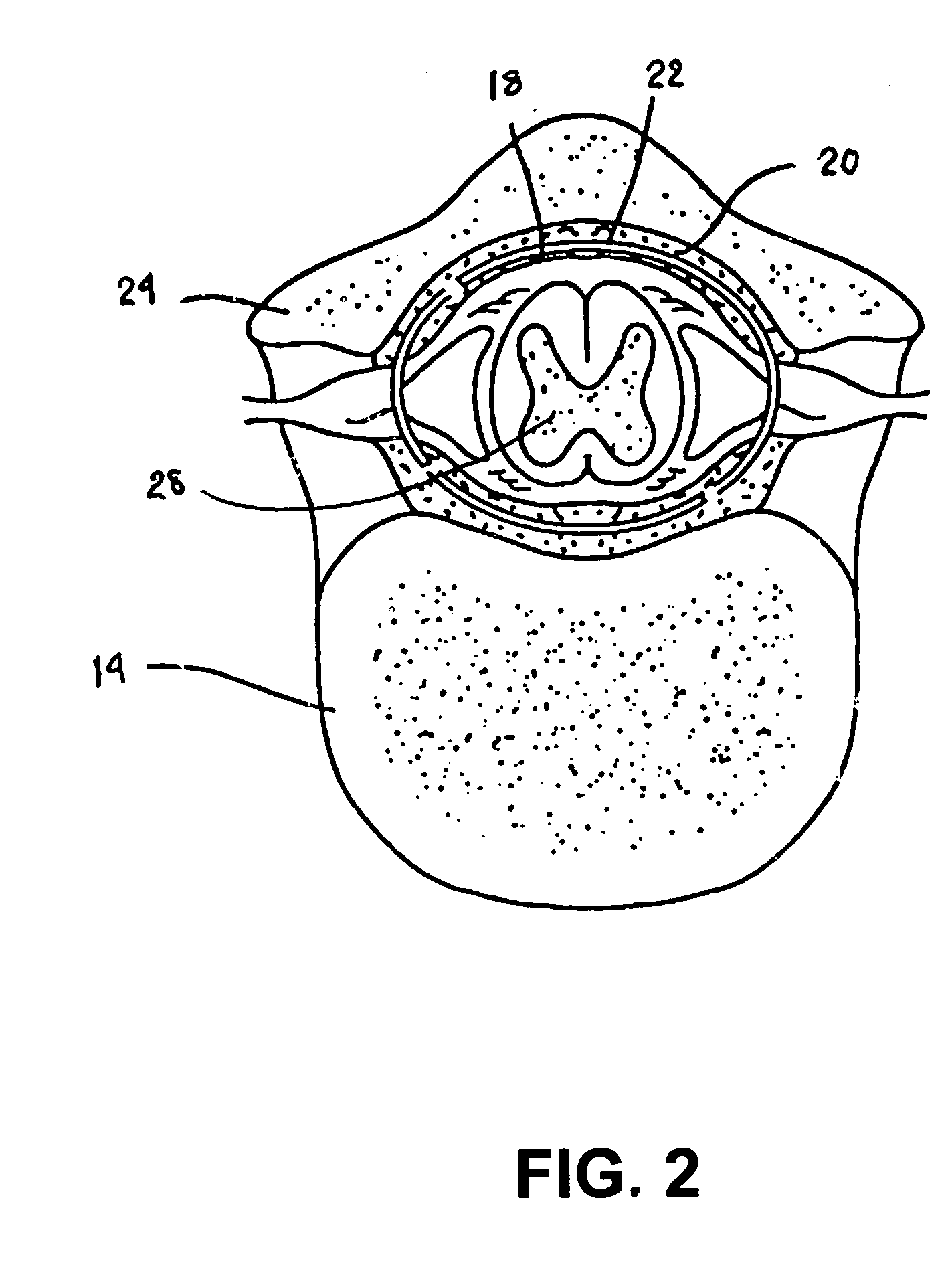
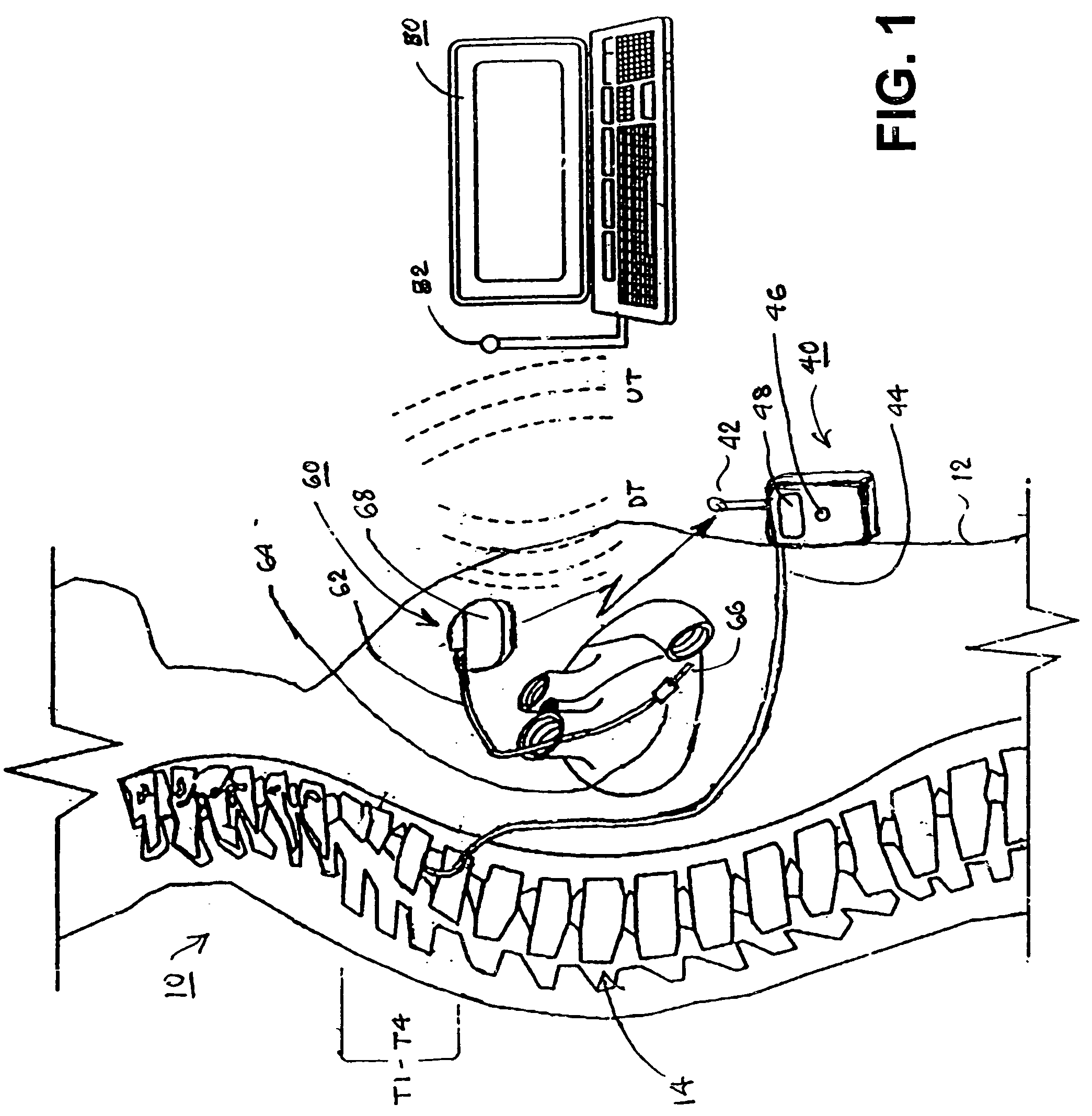
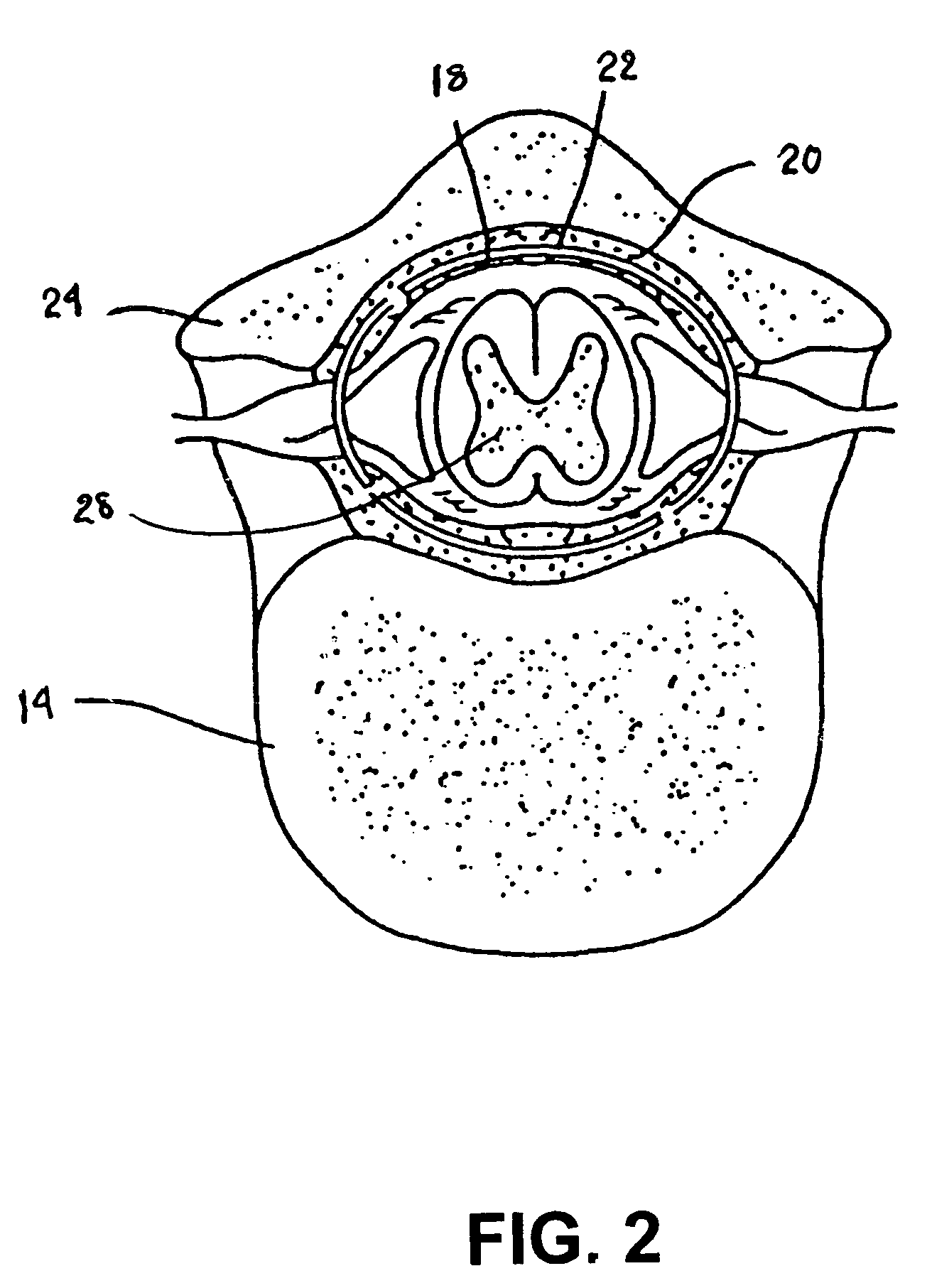
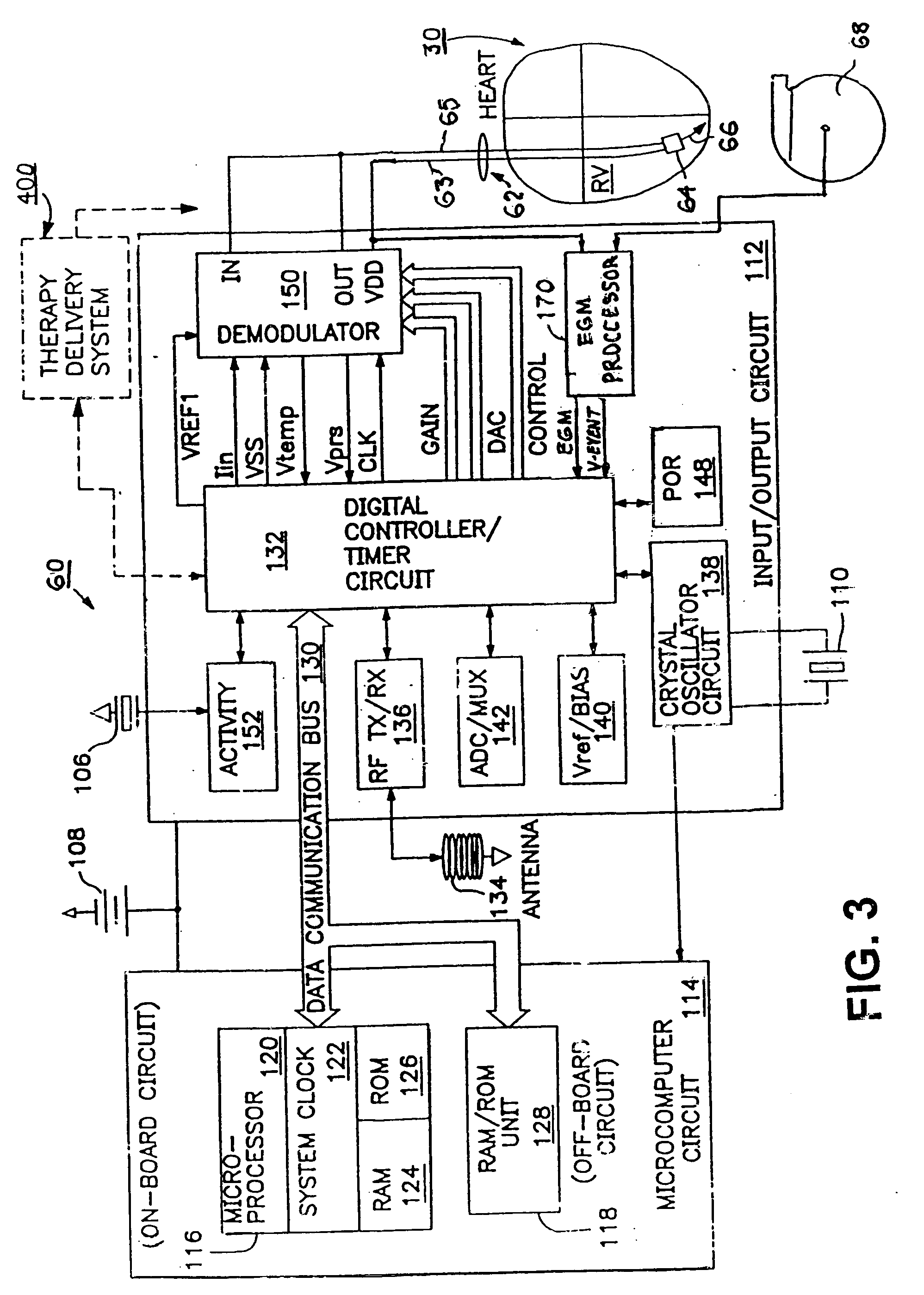
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Pharmaceutical Compositions and Related Methods of Treatment
InactiveUS20080021074A1Decrease and prevent sleepinessDecrease and prevent and lethargyBiocideNervous disorderAdrenergic receptor agonistsClumsiness
Pharmaceutical compositions comprising at least one alpha2-adrenergic agonist or baclofen and at least one alpha1-adrenergic agonist are disclosed. Pharmaceutical compositions comprising tizanidine and modafinil are disclosed. Methods for reducing somnolence, sleepiness, lethargy, dizziness, drowsiness, somnolence, tiredness, lightheadedness, increased weakness, confusion, unsteadiness, clumsiness, or a combination of the symptoms thereof in a human patient; treating pain; and attenuating muscle spasticity, using pharmaceutical compositions comprising at least one alpha2-adrenergic agonist or baclofen and at least one alpha1-adrenergic agonist are disclosed.
Owner:QUESTCOR PHARMA
Delivery of a sympatholytic cardiovascular agent to the central nervous system
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
Extended release alpha-2 agonist pharmaceutical dosage forms
InactiveUS20050118256A1Sufficient effectFacilitated releaseBiocideDigestive systemAdrenergic receptor agonistsAgonist drugs
Disclosed is an extended release pharmaceutical formulation containing at least an alpha-2 adrenergic agonist, such as tizanidine, for the treatment and prevention of spasticity in a subject, e.g., painful inflammatory conditions associated with skeletal muscle spasms.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Pharmaceutical formulation containing muscle relaxant and COX-II inhibitor
InactiveUS20050100594A1Minimize side effectsMaintain curative effectBiocidePill deliveryCyclooxygenaseSkeletal muscle
Disclosed is an extended release pharmaceutical formulation comprising a muscle relaxant drug, such as tizanidine, in combination with a cyclooxygenase-2 inhibitor, such as valdecoxib. The formulations are useful in the treatment and management of painful inflammatory conditions associated with, for example, skeletal muscle spasms.
Owner:GLENMARK PHARMACEUTICALS LIMITED
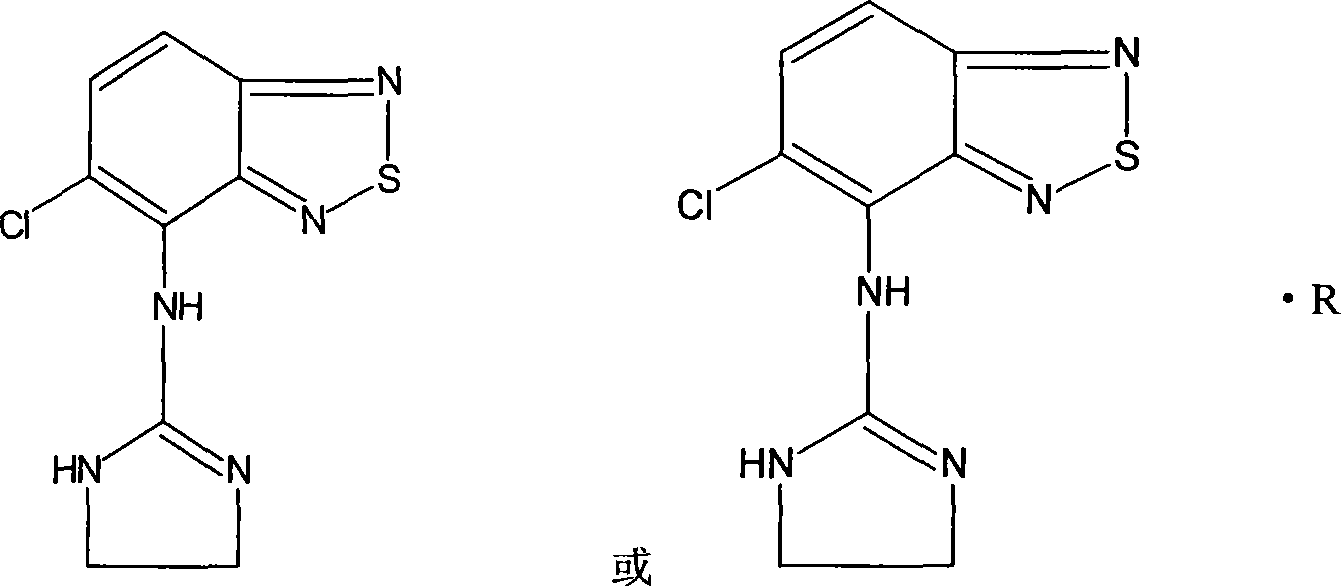
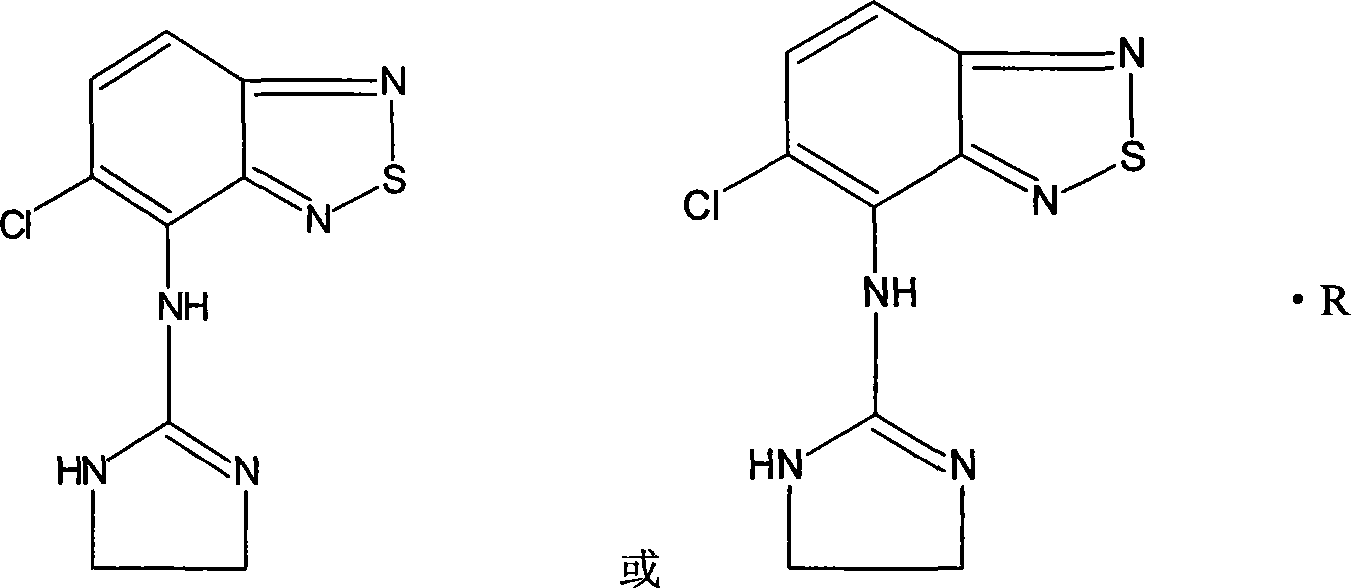
Novel use of tizanidine or its derivatives
ActiveCN101045051ANo dependencyNo withdrawal reactionOrganic active ingredientsNervous disorderSleep durationInsomnia
An application of tizanidine or its derivative in preparing the medicines for treating insomnia, elongating SWS sleeping and preventing senile dementia is disclosed.
Owner:SICHUAN CREDIT PHARMA
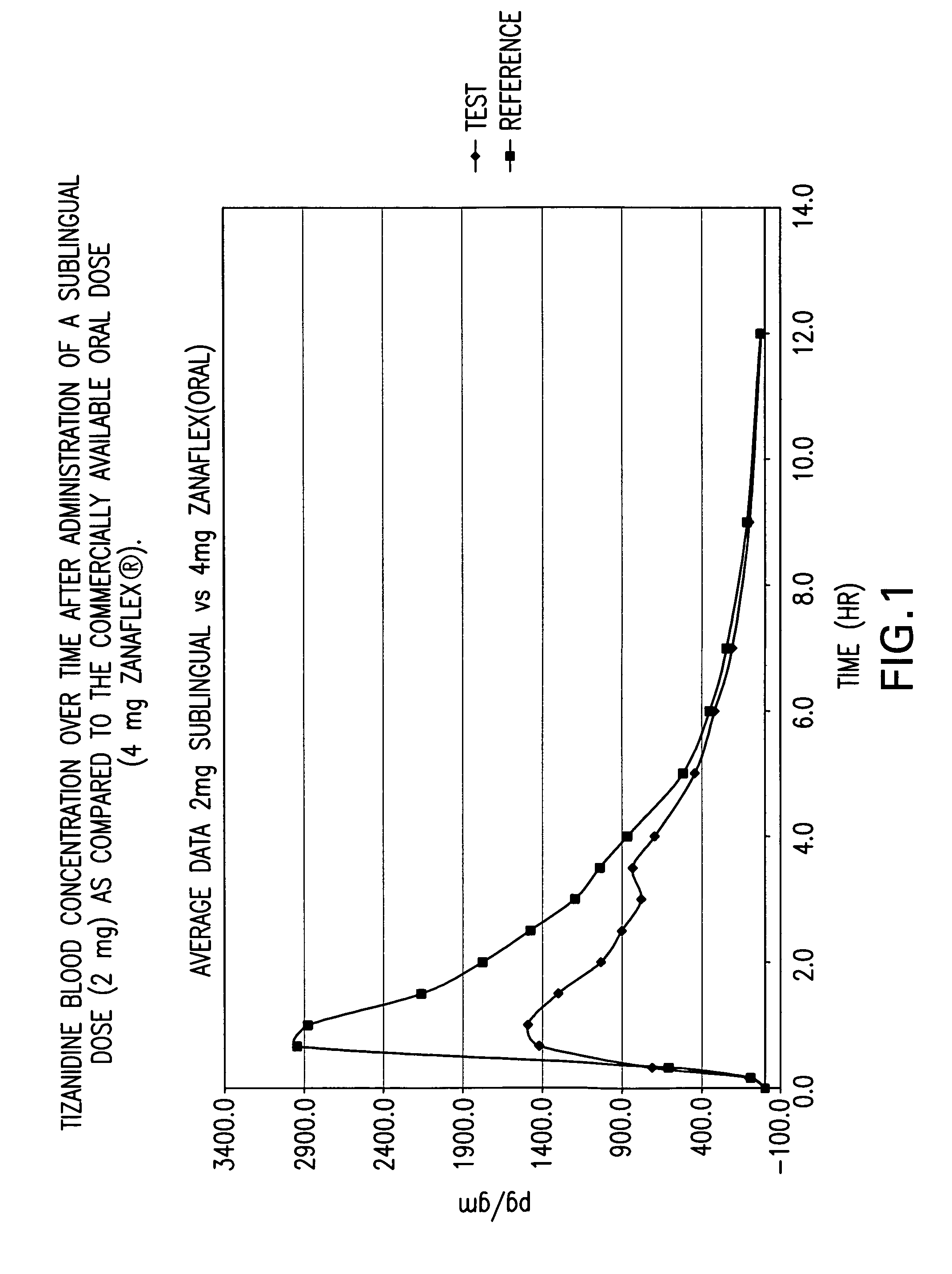
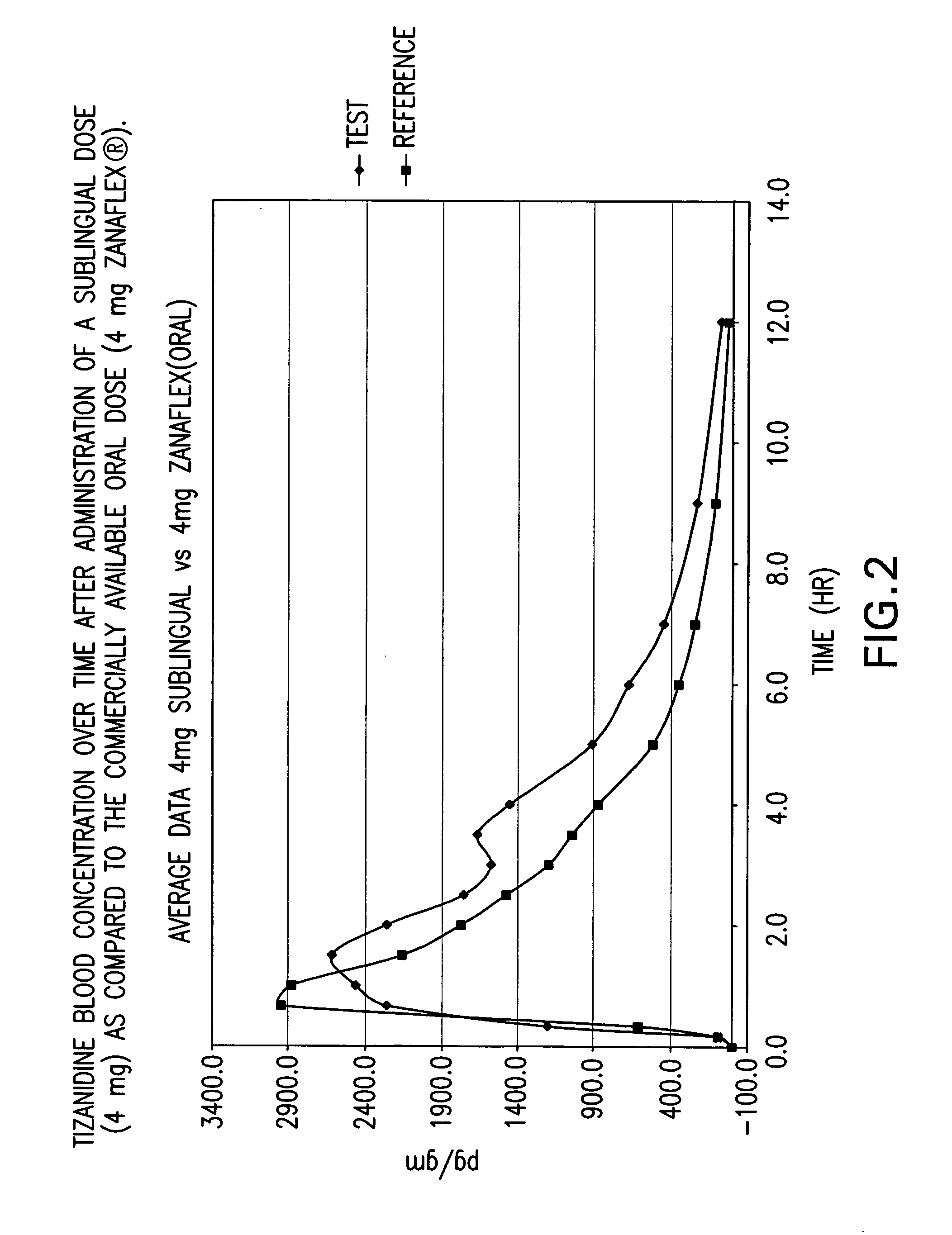
Tizanidine compositions and methods of treatment using the compositions
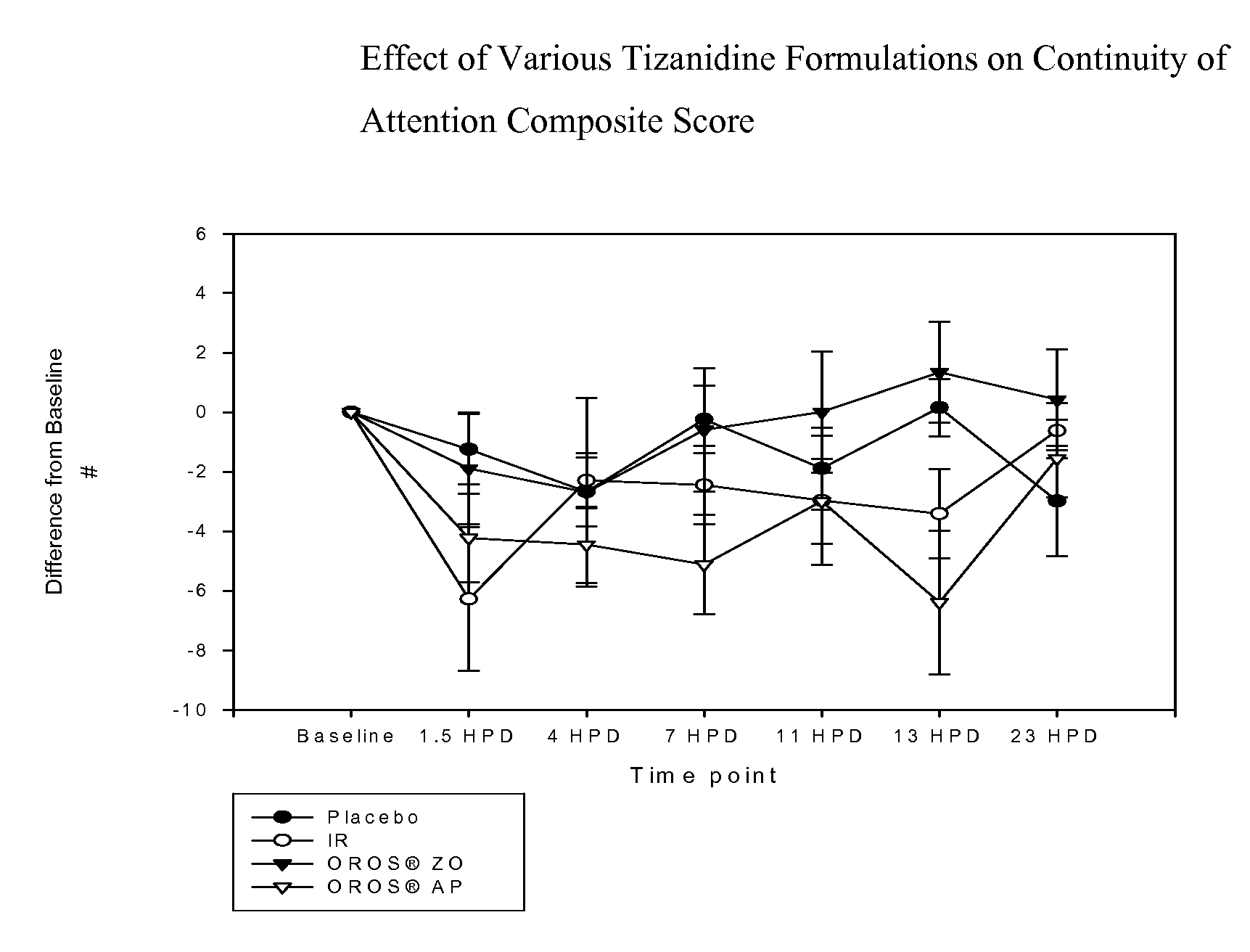
InactiveUS20070078174A1Improving daytime quality of lifeImprove sleep qualityBiocideNervous disorderBlood concentrationNervous system
The invention is directed to methods of treating spasticity in patient having a neurological disease comprising administering to a patient in need of such treatment a tizanidine formulation providing a tizanidine blood concentration of at least about 900 pg / ml for about five hours, wherein the formulation is administered prior to bedtime.
Owner:TEVA PHARM USA INC
Zero order controlled release compositions of tizanidine
InactiveUS20080194655A1Eliminate side effectsBiocidePill deliveryOrder controlControlled-Release Formulations
The present invention relates to a novel controlled release formulations of tizanidine. The invention also provides methods of using novel controlled release formulations of tizanidine to treat a patient.
Owner:ALZA CORP
Controlled release compositions of tizanidine
ActiveUS20080214629A1Increasing plasma tizanidine concentrationBiocideMuscular disorderControlled-Release FormulationsTizanidine
The present invention relates to a novel controlled release formulations of tizanidine. The invention also provides methods of using novel controlled release formulations of tizanidine to treat a patient.
Owner:ALZA CORP
Implantable tizanidine compositions and methods of treatment thereof
Owner:BRAEBURN PHARMA INC
Implantable tizanidine compositions and methods of treatment thereof
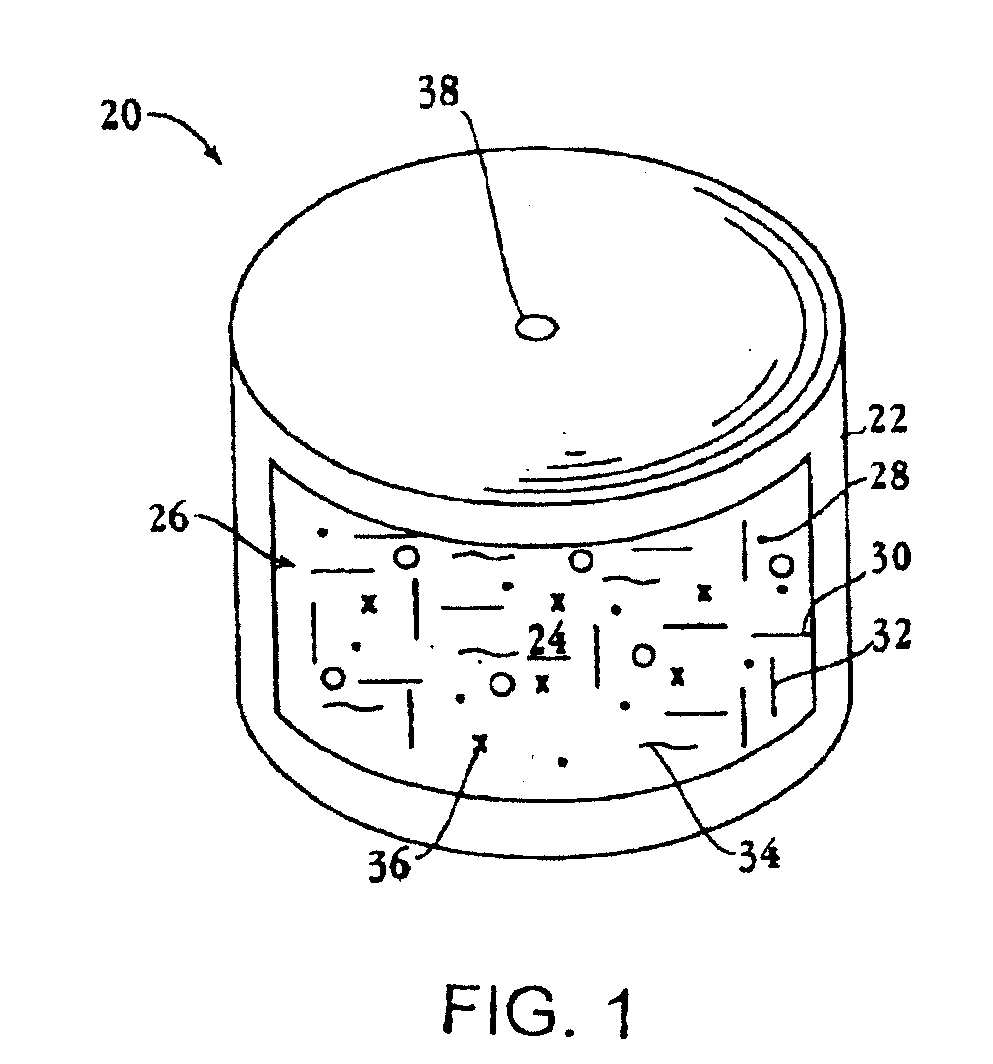
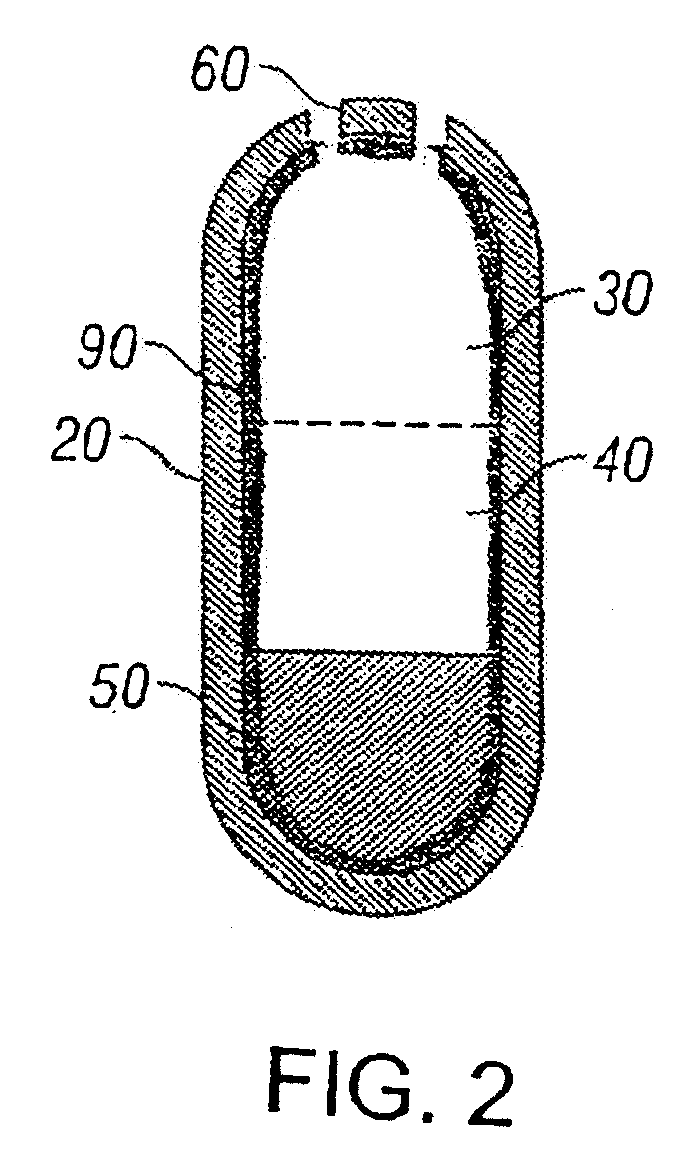
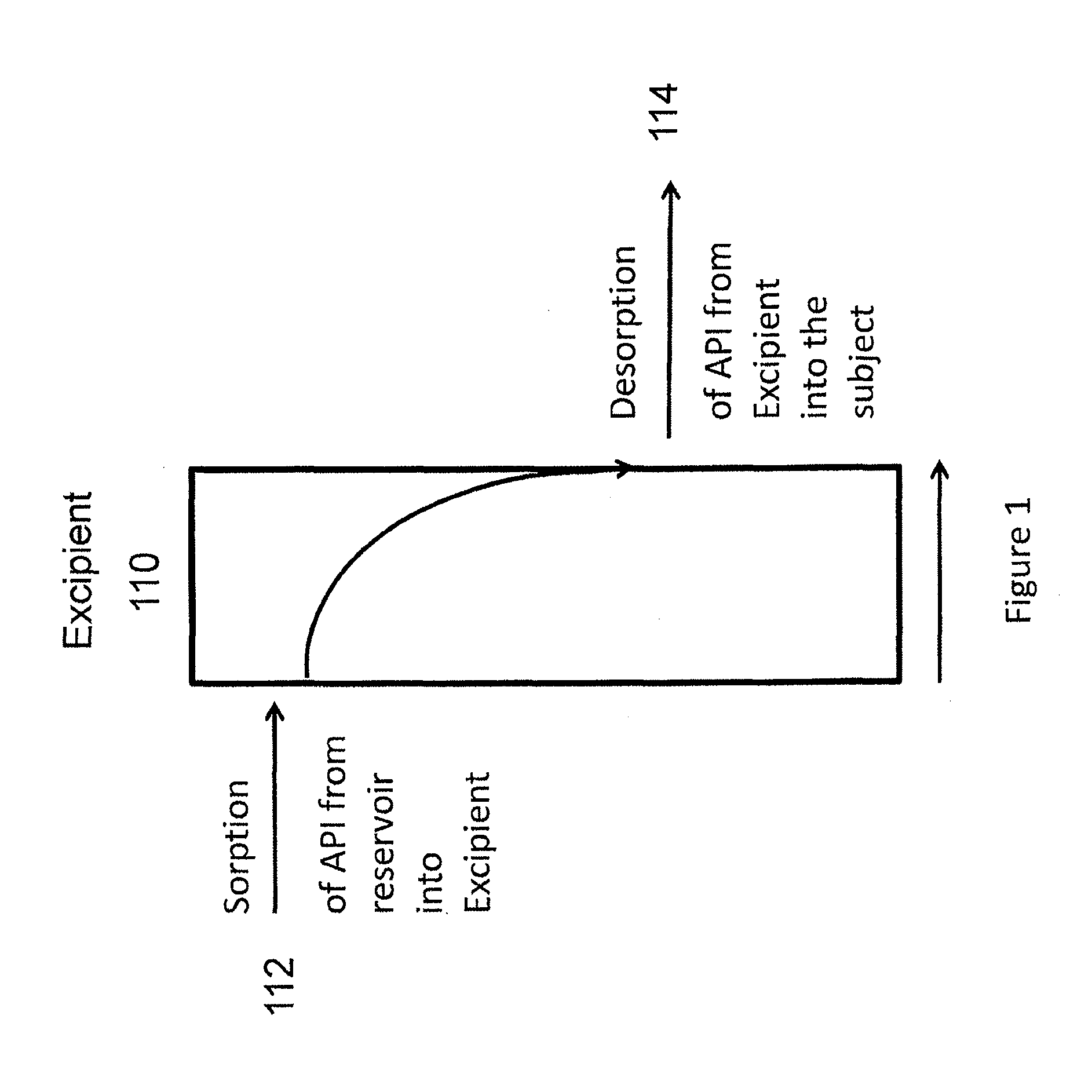
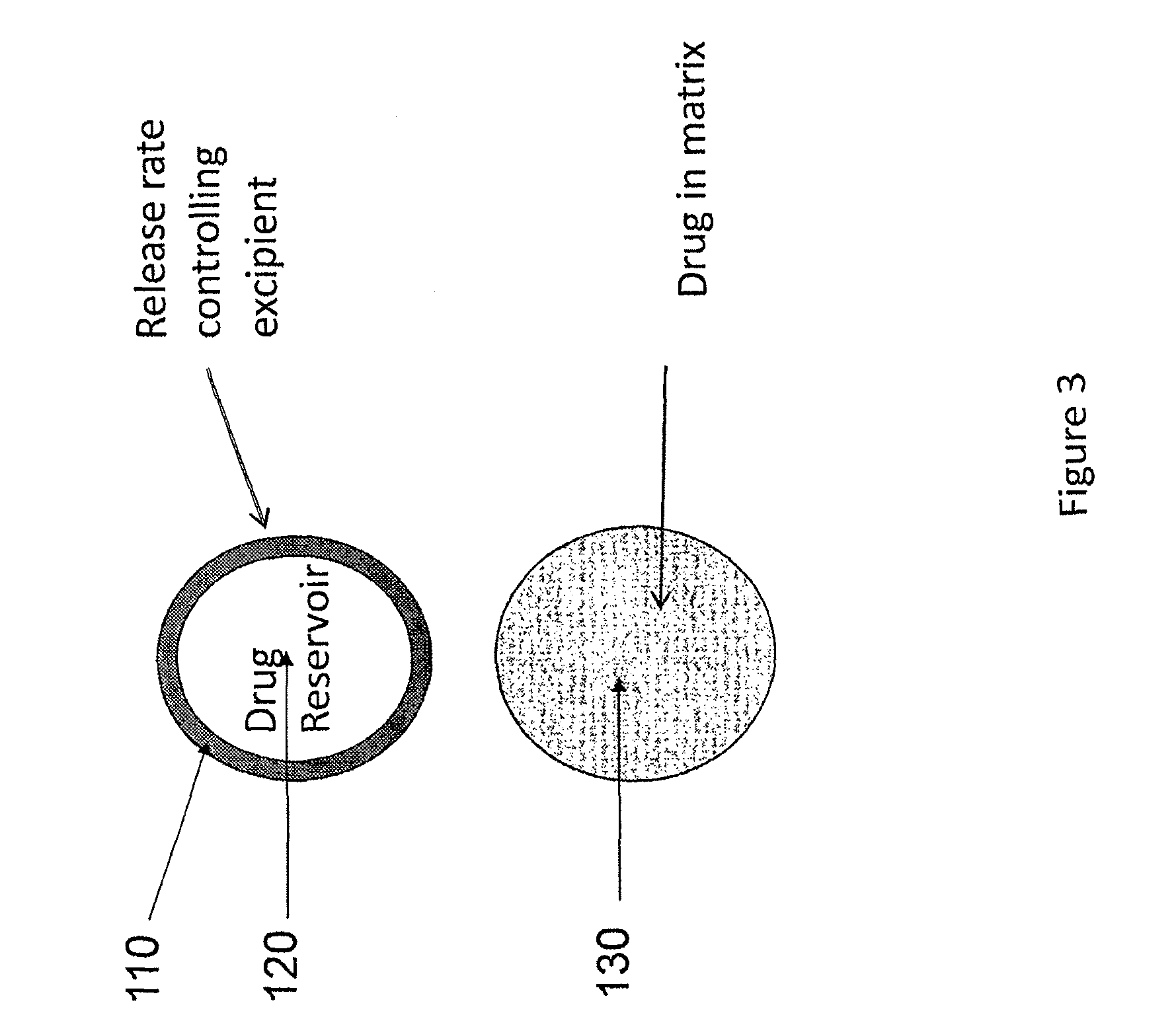
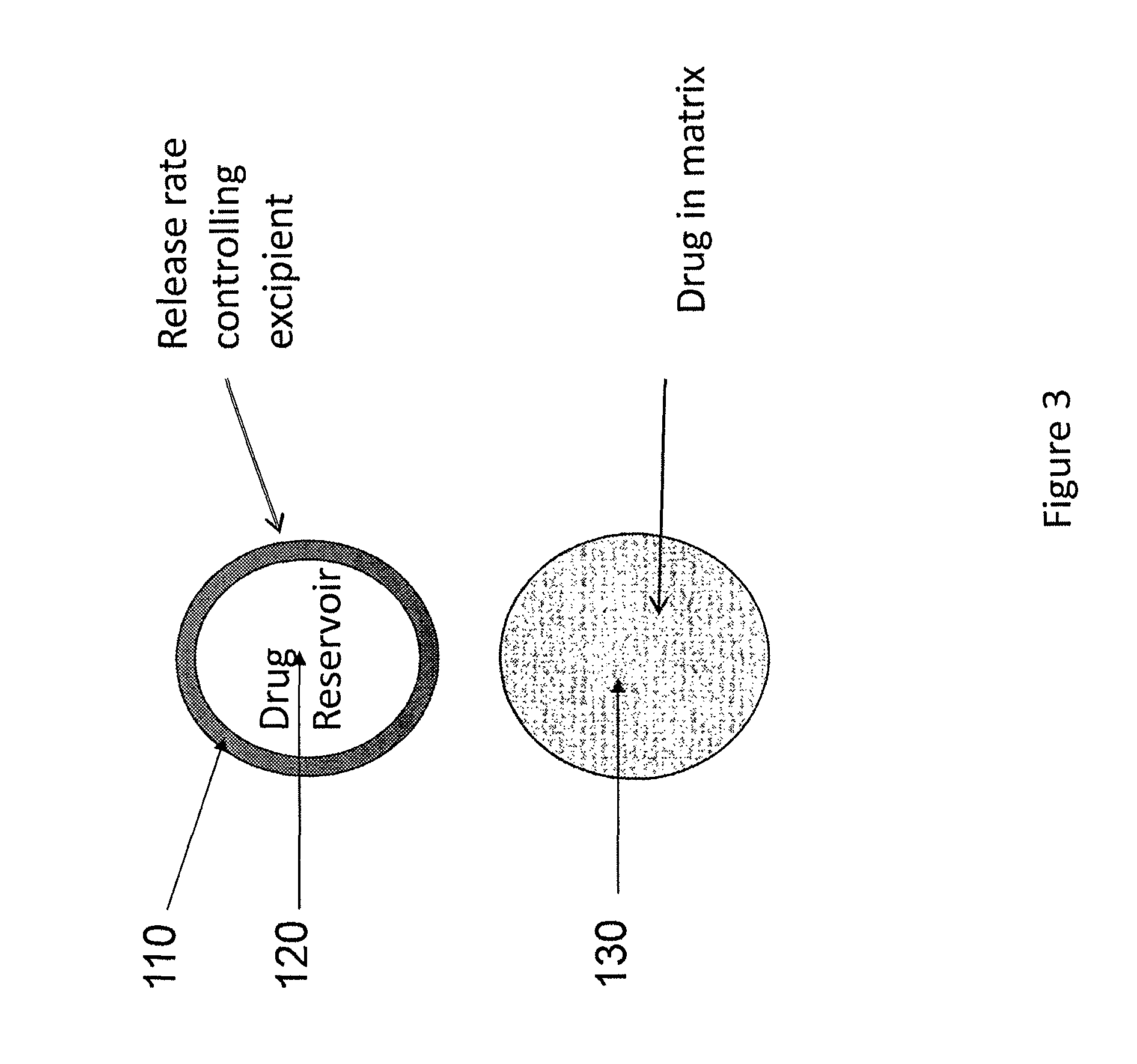
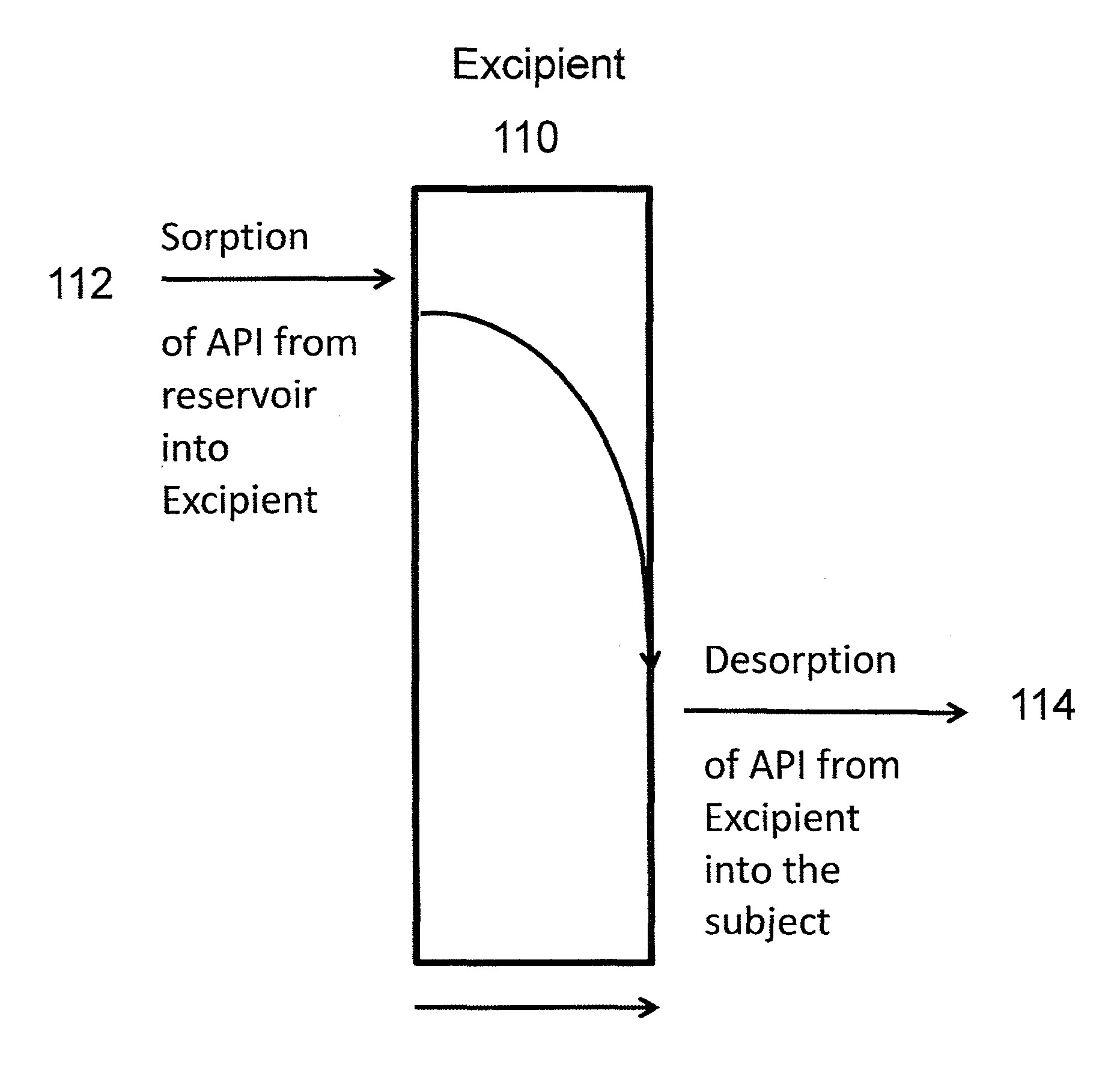
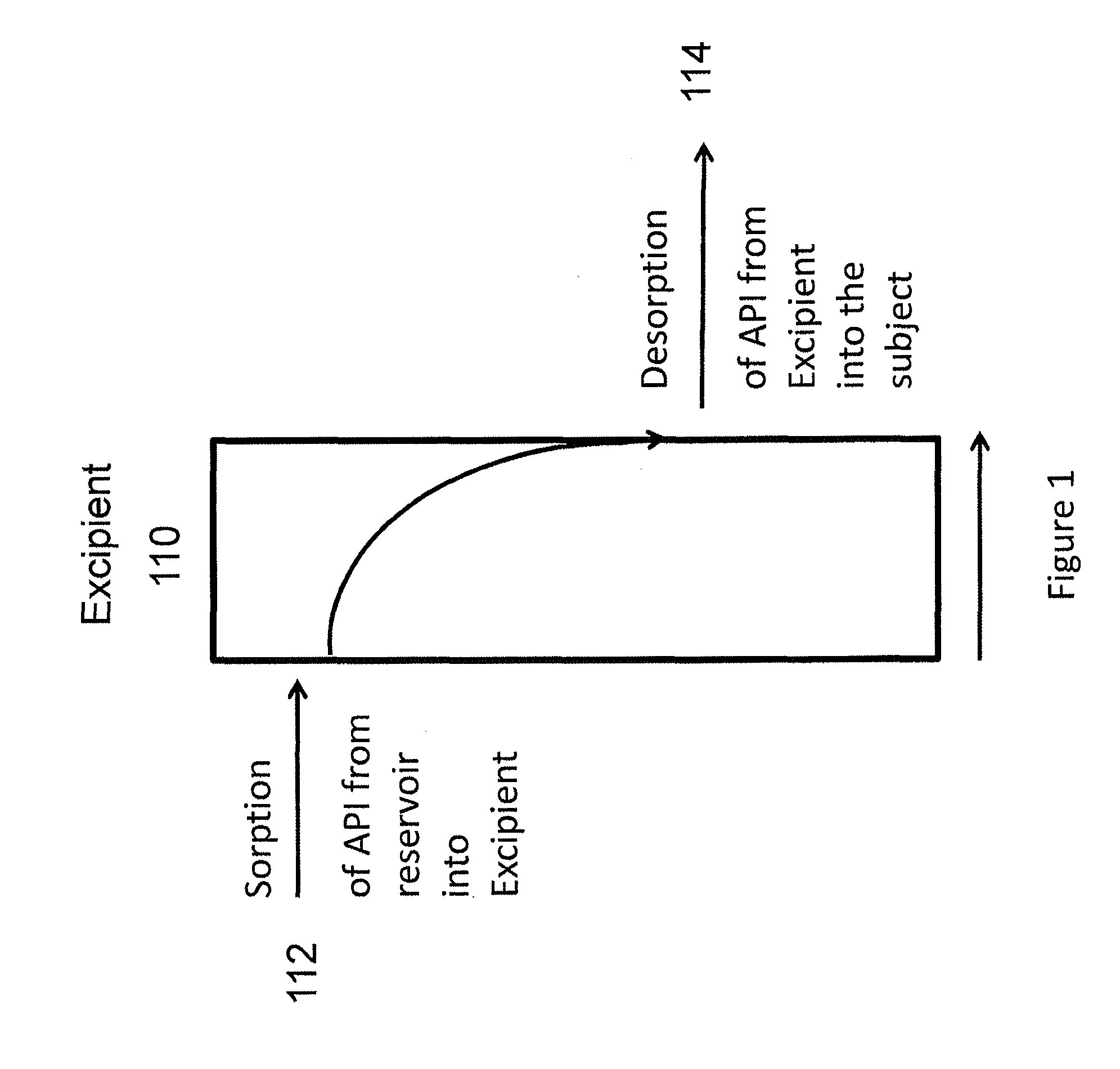
A method of treating the symptoms of spasticity comprises implanting a reservoir-based drug delivery composition into a subject to systemically deliver a therapeutically effective amount of tizanidine to the subject for a long period of time (e.g., one month or one year). The drug delivery composition may include a rate-controlling excipient (e.g., an elastomeric polymer) defining a reservoir containing at least one discrete solid dosage form (e.g., one or more pellets), which includes tizanidine free base and optionally, a sorption enhancer.
Owner:BRAEBURN PHARMA INC
Method and compound for treatment of menopausal symptoms
ActiveUS9468631B1Reduces effect menopausal symptomRelieve symptomsOrganic active ingredientsOrganic chemistryHot flashes/flushesDopamine
The subject invention describes a method of use of Ropinirole™ to alleviate and control menopausal symptoms in women, and in particular, hot flashes. The invention describes the use of Ropinirole as a dopamine agonist with affinity for the dopamine D2, D3, or D4 receptors. Ropinirole may also be used to treat menopausal symptoms in conjunction with Tizanidine™ to further reduce the effects menopausal symptoms by providing a sedative and muscle relaxant effect which aids in sleep. The combination of Ropinirole and Tizanidine provides a useful new compound for treatment of menopausal symptoms that are most disruptive to the functioning in activities of daily living.
Owner:KNOBLER ROBERT L
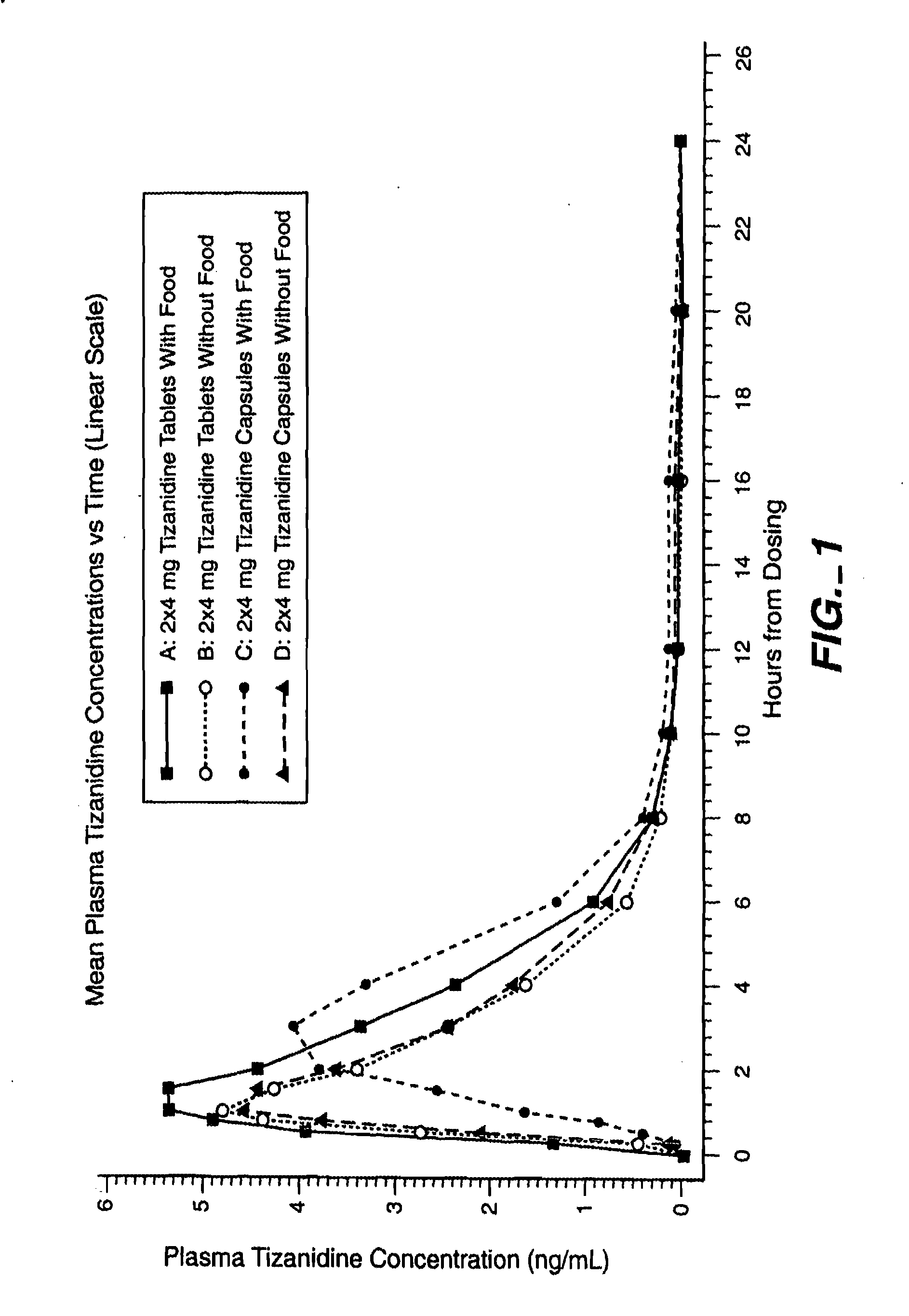
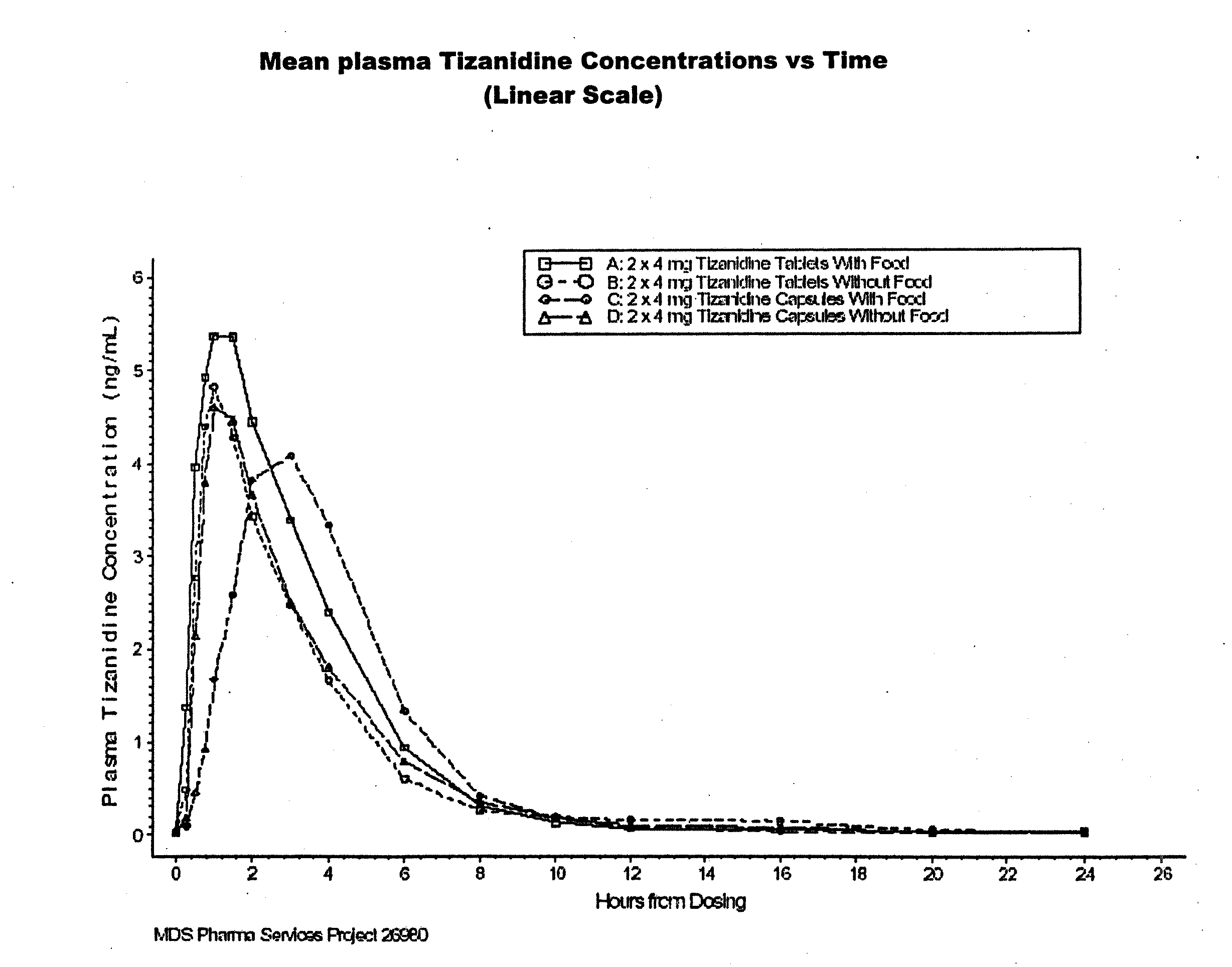
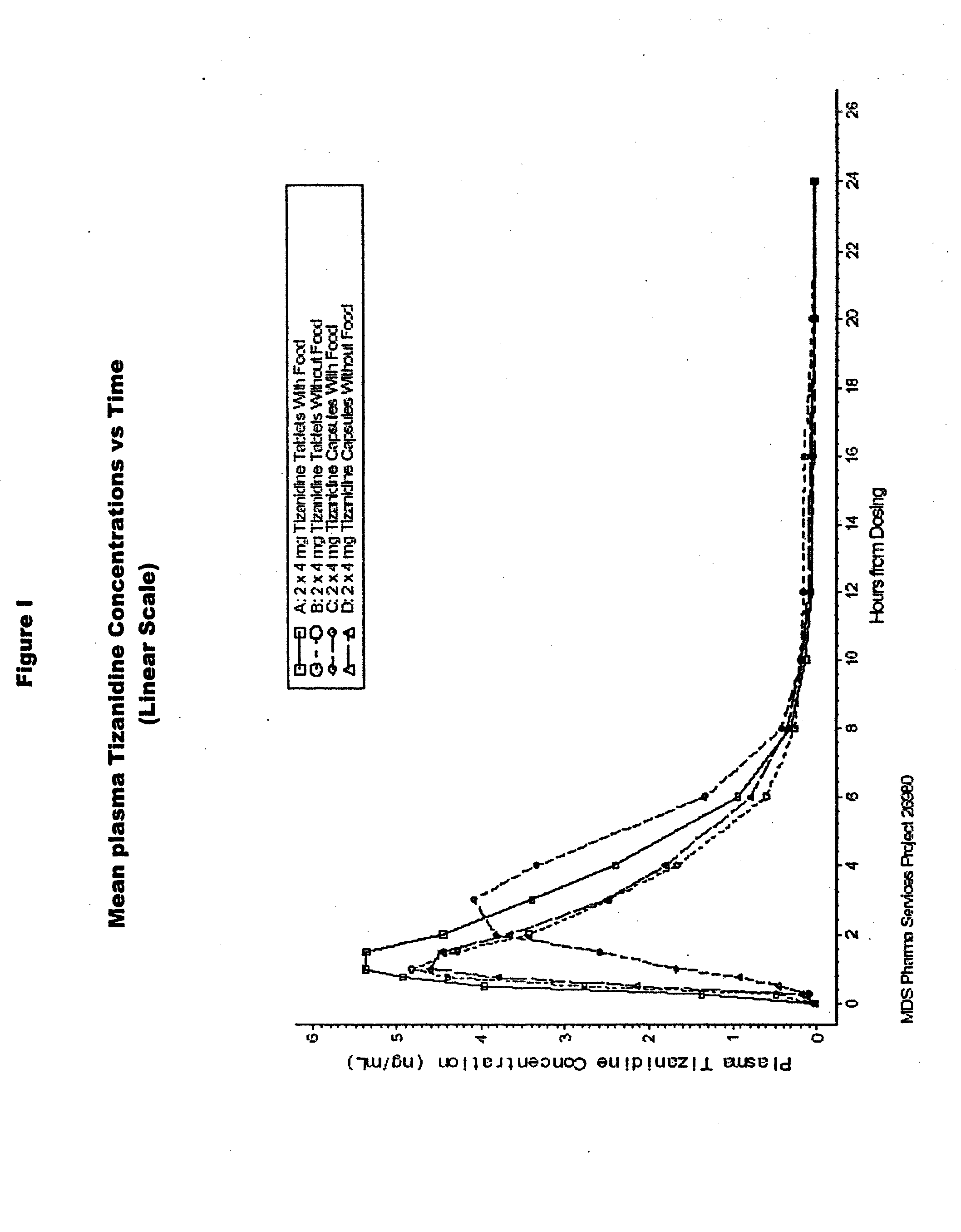
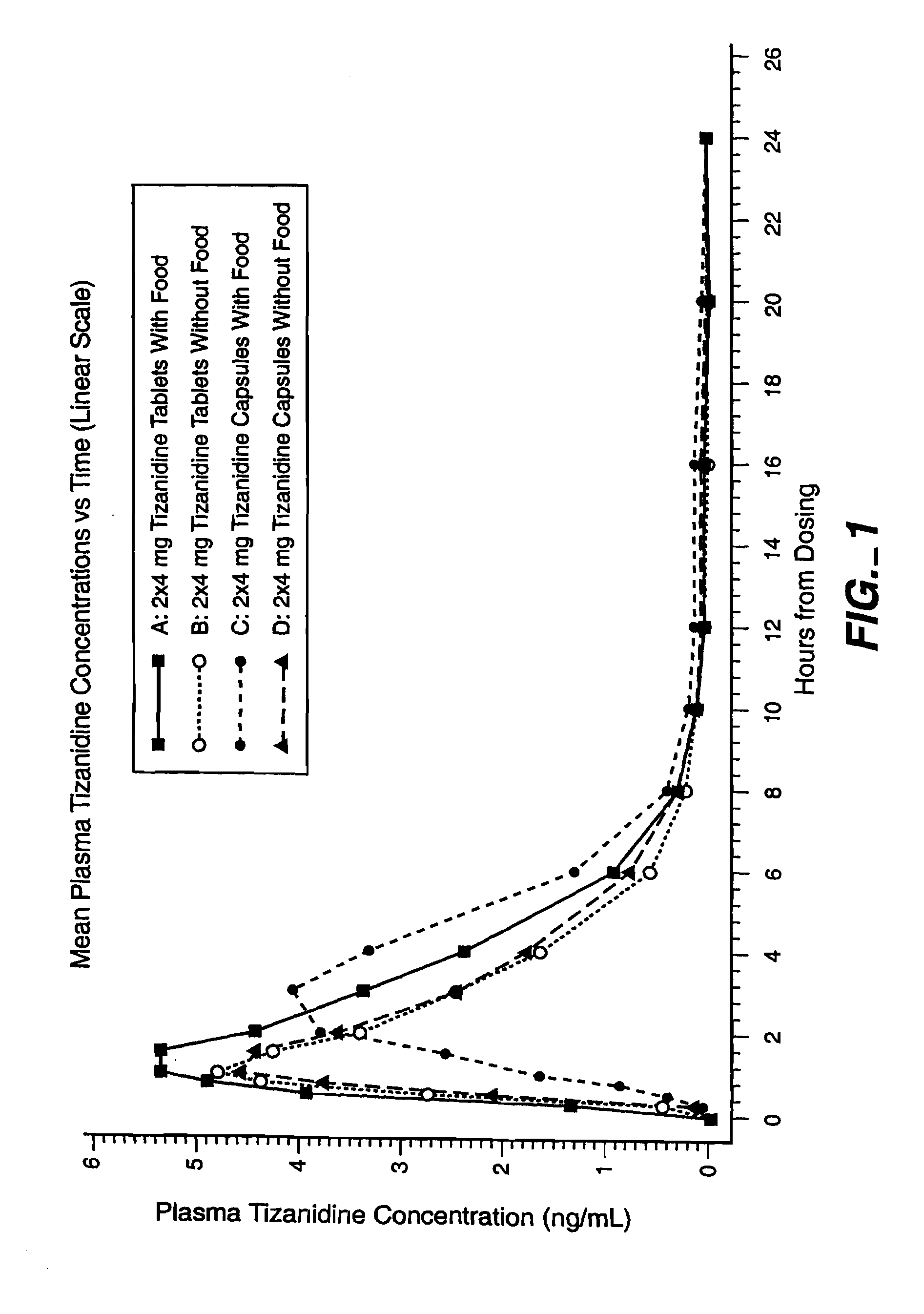
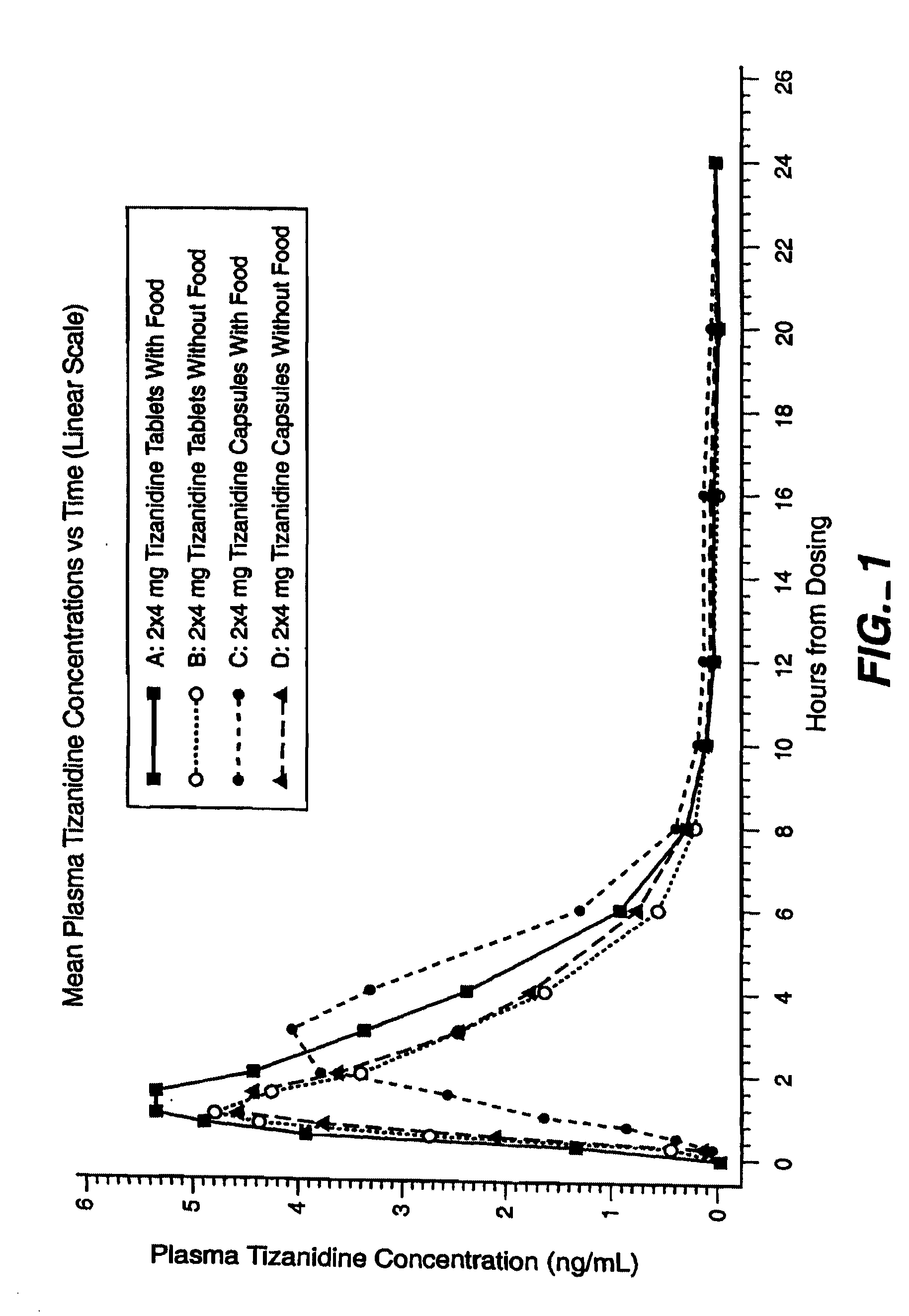
Method of increasing the extent of absorption of tizanidine
An article and method for increasing the extent of tizanidine absorption in a patient receiving tizanidine therapy. Tizanidine may be administered in the form of an immediate release tablet composition at or around the time food is consumed. The composition may be packaged in a container for distribution.
Owner:ACORDA THERAPEUTICS INC
Slow release preparation of tizanidine
InactiveCN101002743AOrganic active ingredientsInorganic non-active ingredientsCurative effectTizanidine
Owner:刘凤鸣
Implantable tizanidine compositions and methods of treatment thereof
A method of treating the symptoms of spasticity comprises implanting a reservoir-based drug delivery composition into a subject to systemically deliver a therapeutically effective amount of tizanidine to the subject for a long period of time (e.g., one month or one year). The drug delivery composition may include a rate-controlling excipient (e.g., an elastomeric polymer) defining a reservoir containing at least one discrete solid dosage form (e.g., one or more pellets), which includes tizanidine free base and optionally, a sorption enhancer.
Owner:BRAEBURN PHARMA INC
Method of reducing somnolence in patients treated with tizanidine
InactiveUS20090017109A1Least overall somnolenceRaise the possibilityBiocidePowder deliveryImmediate releaseDaytime somnolence
An article and method for reducing somnolence in a patient receiving tizanidine therapy. Tizanidine may be administered in the form of an immediate release multiparticulate composition at or around the time food is consumed. The composition may be packaged in a container for distribution.
Owner:PELLEGRINI CARA A +1
Method of reducing somnolence in patients treated with tizanidine
InactiveUS20120027851A1Least overall somnolenceRaise the possibilityBiocidePowder deliveryImmediate releaseDaytime somnolence
An article and method for reducing somnolence in a patient receiving tizanidine therapy. Tizanidine may be administered in the form of an immediate release multiparticulate composition at or around the time food is consumed. The composition may be packaged in a container for distribution.
Owner:KING GEORGE HLDG LUXEMBOURG IIA S A R L
Novel formulation of Tizanidine and derivative thereof and preparation method thereof
ActiveCN1961859BOvercoming hypotensionOrganic active ingredientsMuscular disorderSide effectTherapeutic effect
The invention relates to a nizatidine and it's derivate, wherein it contains the general-release paret whose active compotents are nizatidine and its derivate, and the slow-release part, while the mol ratio between nizatidine and derivate are 5-95:5-95. The invention also provides a relative preparation. The test has proves that its effect is higher than slow-release agent, and its rate of side effect is lower than general-release agent.
Owner:SICHUAN CREDIT PHARMA
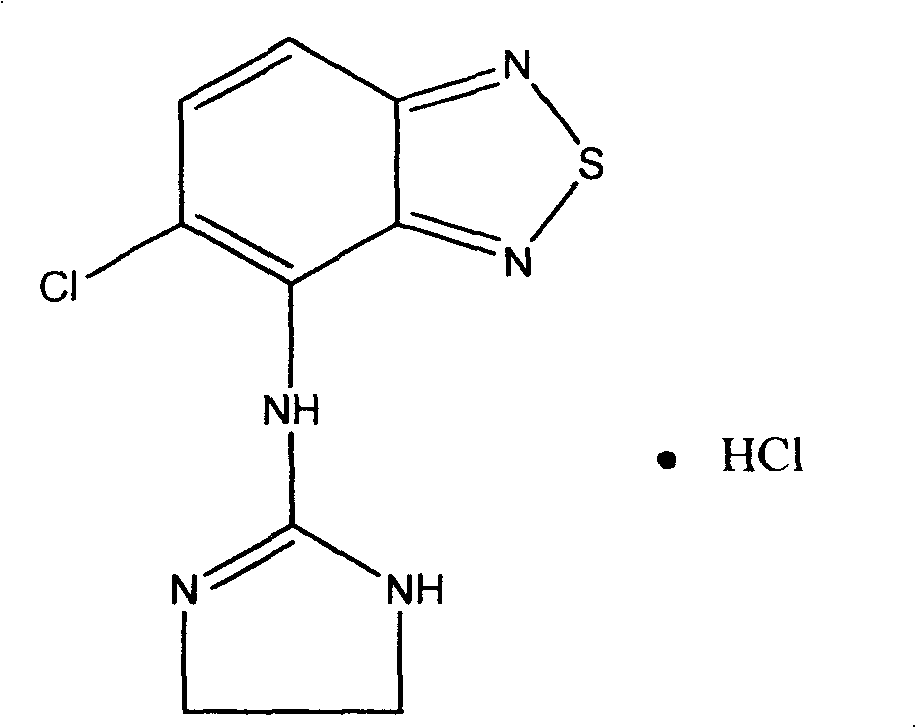
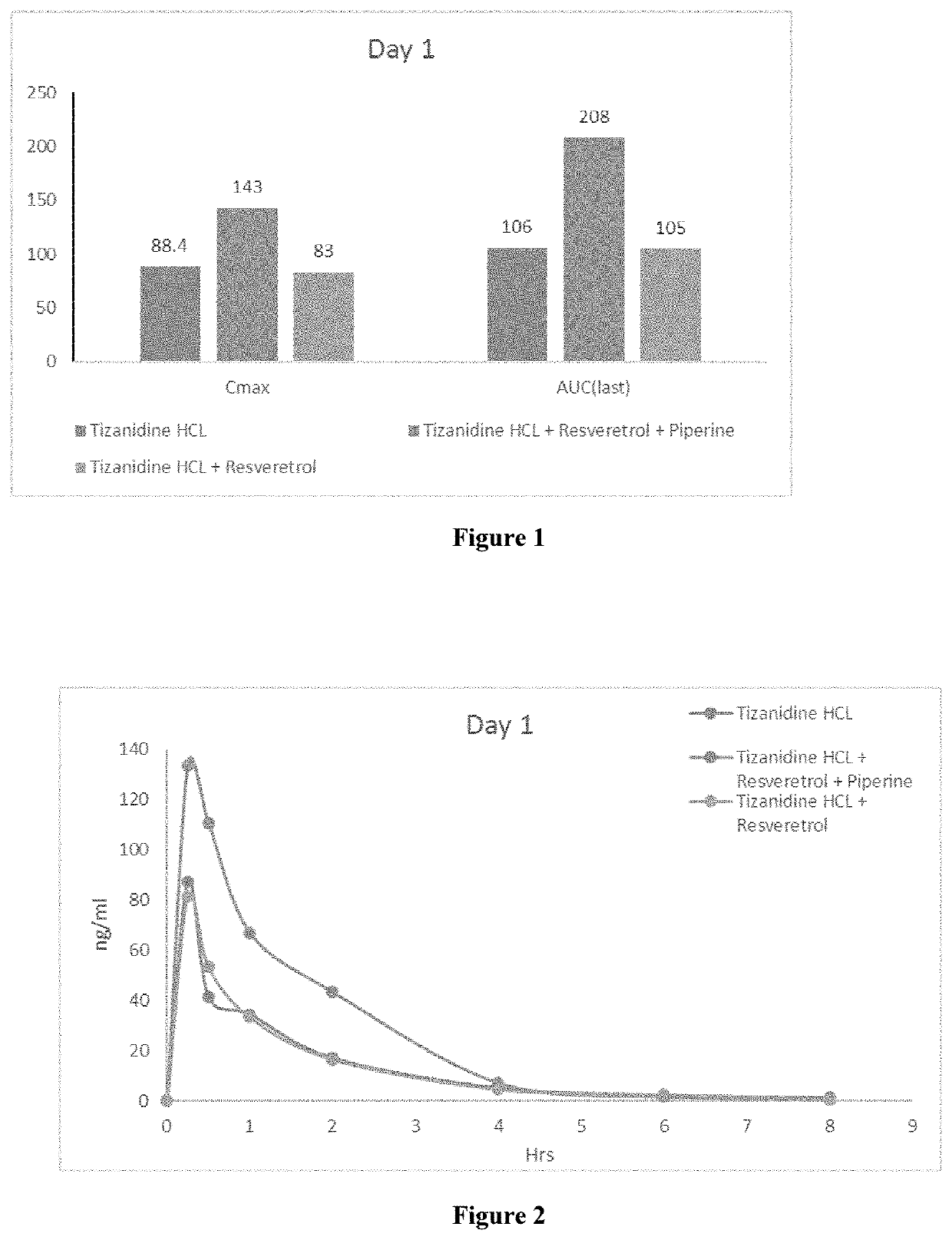
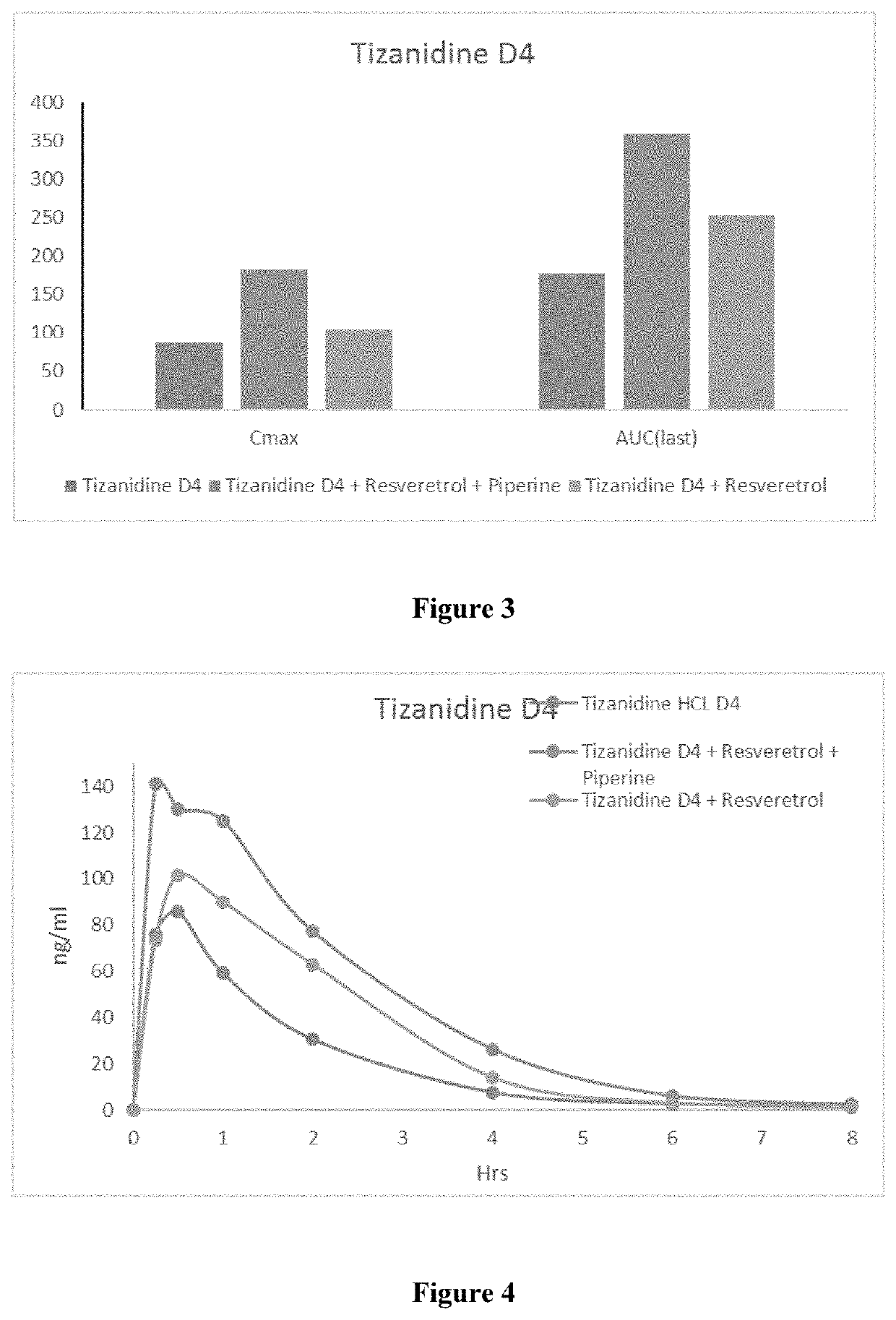
Pharmaceutical Combination Formulations Comprising Tizanidine, Resveratrol and Piperine
PendingUS20220193043A1Increase heightPromote absorptionOrganic active ingredientsDispersion deliveryEfficacyPharmaceutical Substances
The present invention relates to method of increasing the bioavailability / bio-efficacy of tizanidine by co-administering with resveratrol and bioenhancer. The formulation comprising tizanidine, resvetarol and bioenhancer are also provided which can be used for treatment of muscle spasticity.
Owner:CIPLA LTD
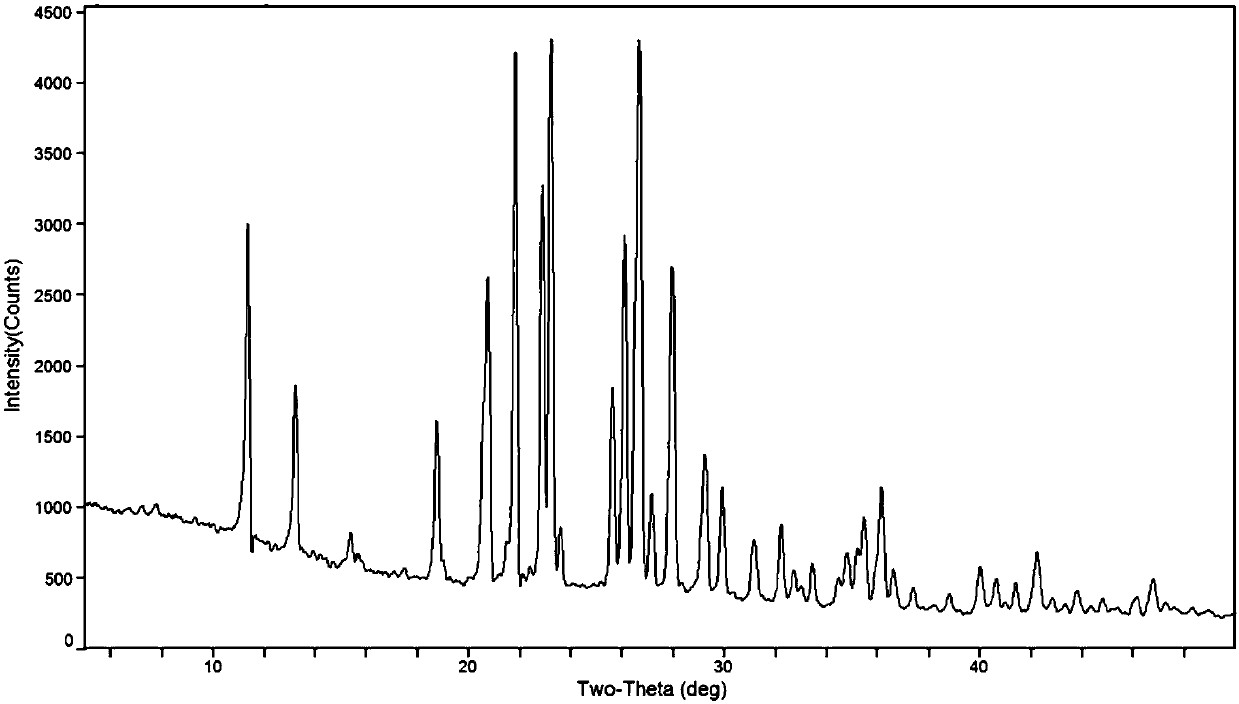
Tizanidine nitrate crystal form A, and preparation method and applications thereof
ActiveCN109535152AHigh chemical purityImprove high temperature stabilityOrganic active ingredientsOrganic chemistry methodsEffective solutionNitrate
The invention provides a tizanidine nitrate crystal form A, and a preparation method and applications thereof. The provided tizanidine nitrate crystal form A uses a Cu-K[alpha] radiation source to perform X-ray powder diffraction, and a 2[theta] diffraction angle has characteristic absorption peaks at 11.4 + / - 0.2, 20.8 + / - 0.2, 21.8 + / - 0.2, 23.2 + / - 0.2 and 26.7 + / - 0.2 degrees; the crystal formA is high in chemical purity, stable in property and low in hygroscopicity, and an effective solution can be provided for the improvement of the availability and security of drugs; and in addition, the crystal form A is simple in preparation technology, short in reaction time and high in product yield, and has no special requirements on equipment, so that the crystal form A is suitable for industrial production.
Owner:SICHUAN CREDIT PHARMA
Novel use of tizanidine or its derivatives in preparing medicine for prolonging fast wave sleep
ActiveCN101045051BFast sleepinessShorten time to sleepOrganic active ingredientsNervous disorderSleep durationInsomnia
An application of tizanidine or its derivative in preparing the medicines for treating insomnia, elongating SWS sleeping and preventing senile dementia is disclosed.
Owner:SICHUAN CREDIT PHARMA
Tizanidine formulations
InactiveUS20190060287A1Reduce the impactEffective levelingOrganic active ingredientsNervous disorderImmediate releaseSedation
The disclosure is directed to pharmaceutical compositions comprising of Tizanidine or a pharmaceutically acceptable salt thereof which controllably modulate the onset of at least one secondary effect associated with Tizanidine administration. In embodiments, the pharmaceutical compositions comprise at least three components with different release characteristics: immediate release component; a first time, pulsatile release component; and a second population of time, pulsatile release component. The release characteristics of the pharmaceutical composition are such that the bedtime blood plasma concentrations control spasticity and allow the patient to have quality sleep, and the daytime blood plasma concentrations controls spasticity without unwanted secondary such as somnolence or sedation.
Owner:ADARE PHARM INC
New use of tizanidine and its derivatives in preparing medicine for treating anxiety disorder
The invention relates to a novel application of both tizanidine and a ramification of tizanidine, in particular to the novel application of both tizanidine and the tizanidine ramification in the process of preparing medicines used for treating anxiety disorder. The invention also provides an application of a medicine compound containing tizanidine and the tizanidine ramification in the process ofpreparing medicines used for treating anxiety disorder. As a central muscle relaxants pain-releasing medicine, tizanidine not only can release the pain but also can solve the anxiety of pain sufferers.
Owner:SICHUAN CREDIT PHARMA
Pharmaceutical composition of tizanidine and process for preparing the same
The present invention relates to a solid oral pharmaceutical composition comprising an effective amount of Tizanidine or its pharmaceutically acceptable salts, esters, solvates, polymorphs, stereoisomers or mixtures thereof. The solid oral dosage form of the present invention is free of non-pareil seeds. The invention also relates to a process for the preparation of a pharmaceutical composition comprising an effective amount of Tizanidine wherein, the dosage form is free of non-pareil seeds.
Owner:JUBILANT GENERICS
Method of increasing the extent of absorption of tizanidine
InactiveUS20120108643A1Improve absorptionRemarkable effectBiocideNervous disorderImmediate releaseTizanidine
An article and method for increasing the extent of tizanidine absorption in a patient receiving tizanidine therapy. Tizanidine may be administered in the form of an immediate release tablet composition at or around the time food is consumed. The composition may be packaged in a container for distribution.
Owner:KING GEORGE HLDG LUXEMBOURG IIA S A R L
Tizanidine p-toluenesulfonate crystal form A and preparation method and applications thereof
ActiveCN109535151AHigh chemical purityImprove high temperature stabilityOrganic active ingredientsOrganic chemistry methodsEffective solutionX-ray
The invention provides a tizanidine p-toluenesulfonate crystal form A and a preparation method thereof, and applications. The provided tizanidine p-toluenesulfonate crystal form A uses a Cu-K[alpha] radiation source to perform X-ray powder diffraction, and a 2[theta] diffraction angle has characteristic absorption peaks at 6.7 + / - 0.2, 13.5 + / - 0.2, 20.4 + / - 0.2, 21.4 + / - 0.2 and 24.3 + / - 0.2 degrees. The crystal form A has high product chemical purity, stable properties and low hygroscopicity, and effective solutions can be provided for the improvement of the security and validity of drugs; and in addition, the crystal form A is simple in preparation technology, short in reaction time, high in yield, good in reproducibility and suitable for industrial production.
Owner:SICHUAN CREDIT PHARMA
Method and compound for treatment of menopausal symptoms
ActiveUS9616123B1Relieve symptomsImprove sleepingOrganic active ingredientsOrganic chemistryDopamineMuscle relaxant
Owner:KNOBLER ROBERT L
Method of reducing somnolence in patients treated with tizanidine
InactiveUS20130236539A1Least overall somnolenceRaise the possibilityBiocideNervous disorderImmediate releaseDaytime somnolence
An article and method for reducing somnolence in a patient receiving tizanidine therapy. Tizanidine may be administered in the form of an immediate release multiparticulate composition at or around the time food is consumed. The composition may be packaged in a container for distribution.
Owner:KING GEORGE HLDG LUXEMBOURG IIA S A R L
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com