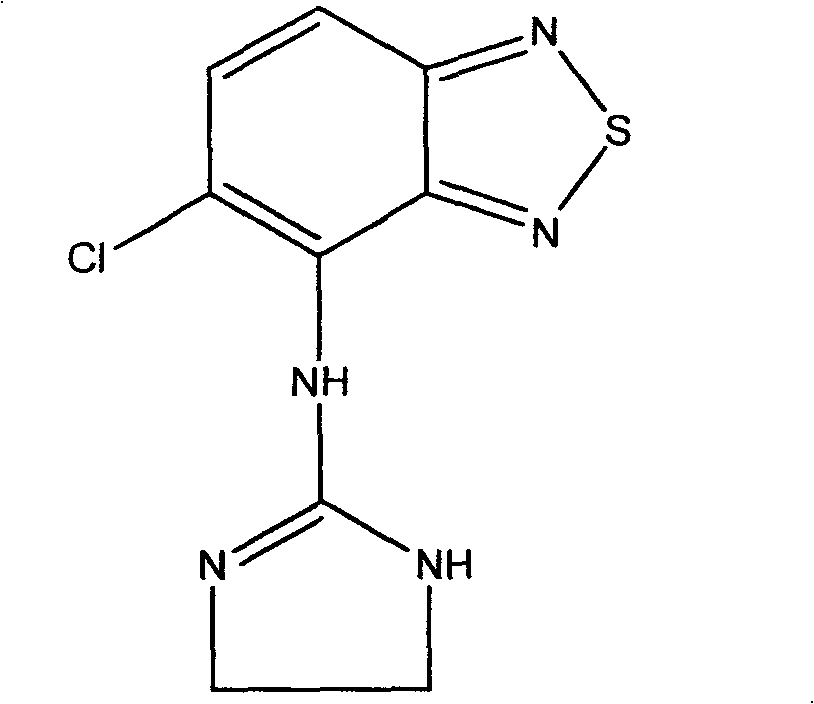
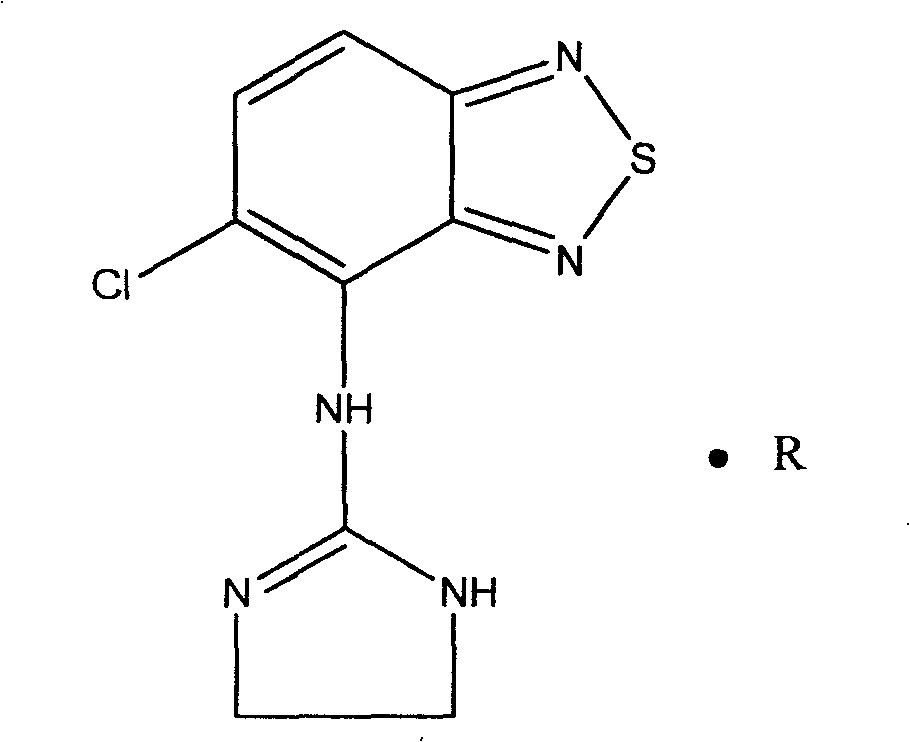
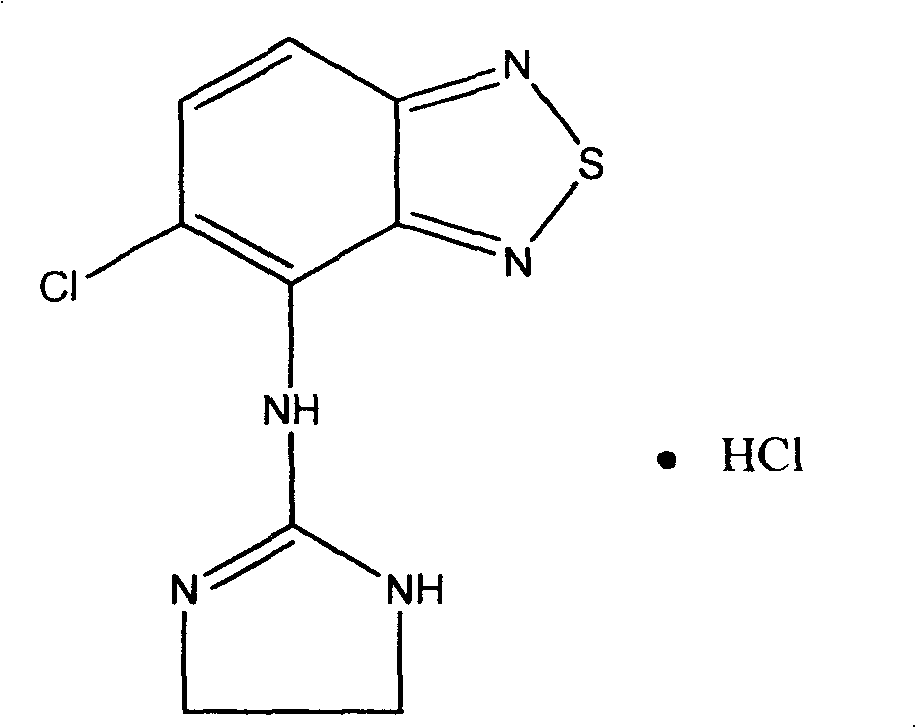
Novel formulation of Tizanidine and derivative thereof and preparation method thereof
A technology of tizanidine and its derivatives, which can be applied to medical preparations containing active ingredients, pill delivery, pharmaceutical formulations, etc., and can solve problems such as high risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1 tablet of the present invention (tizanidine molar ratio 70: 30 in the regular release part and the sustained release part)
[0045] Raw materials: tizanidine hydrochloride 2g, hydroxypropyl methylcellulose (HPMC K4M) 64.8g, lactose 110g, starch 100g, microcrystalline cellulose 5g, 75% ethanol appropriate amount (1000 pieces), the preparation method is as follows:
[0046] A, get tizanidine hydrochloride 0.6g, add hydroxypropyl methylcellulose (HPMC K4M) and lactose, be prepared into sustained-release part inner layer tablet core;
[0047] B. Get 1.4g of tizanidine hydrochloride, add microcrystalline cellulose to make the constant release part, and coat the outer layer of the tablet core obtained in step a to obtain a coating;
[0048]c. Take the coating obtained in step b, add starch and press into tablets according to the conventional method.
Embodiment 2
[0049] Embodiment 2 Preparation of tablet of the present invention (tizanidine molar ratio 65: 35 in the regular release part and the sustained release part)
[0050] Raw materials: 4g of tizanidine hydrochloride, 20g of tragacanth gum, 1.5g of flavoring agent, appropriate amount of distilled water, 80g of starch, 20g of dextrin (1000 tablets), the preparation method is as follows:
[0051] a. Get 1.4 g of tizanidine hydrochloride, add tragacanth gum, and prepare the slow-release part inner layer tablet core;
[0052] B. Get 2.6g of tizanidine hydrochloride, add dextrin to make a constant release part, and coat the outer layer of the tablet core obtained in step a to obtain a coating;
[0053] c. Take the coating obtained in step b, add starch and flavoring agent, and press into tablets according to the conventional method.
Embodiment 3
[0054] Embodiment 3 Preparation of tablet of the present invention (tizanidine molar ratio 75: 25 in the regular release part and the sustained release part)
[0055] Raw materials: 4g of tizanidine hydrochloride, 50g of hydroxypropyl methylcellulose (HPMC K100M), 45g of methylcellulose, 80g of starch, 20g of microcrystalline cellulose (1000 pieces), the preparation method is as follows:
[0056] a, get tizanidine hydrochloride 1.0g, add hydroxypropyl methylcellulose (HPMC K100M), methylcellulose and starch, be prepared into sustained-release part inner layer tablet core;
[0057] B. Get 3.0 g of tizanidine hydrochloride, add microcrystalline cellulose to make a constant release part, and coat the outer layer of the tablet core obtained in step a to obtain a coating;
[0058] c. Take the coating obtained in step b, add starch and press into tablets according to the conventional method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com