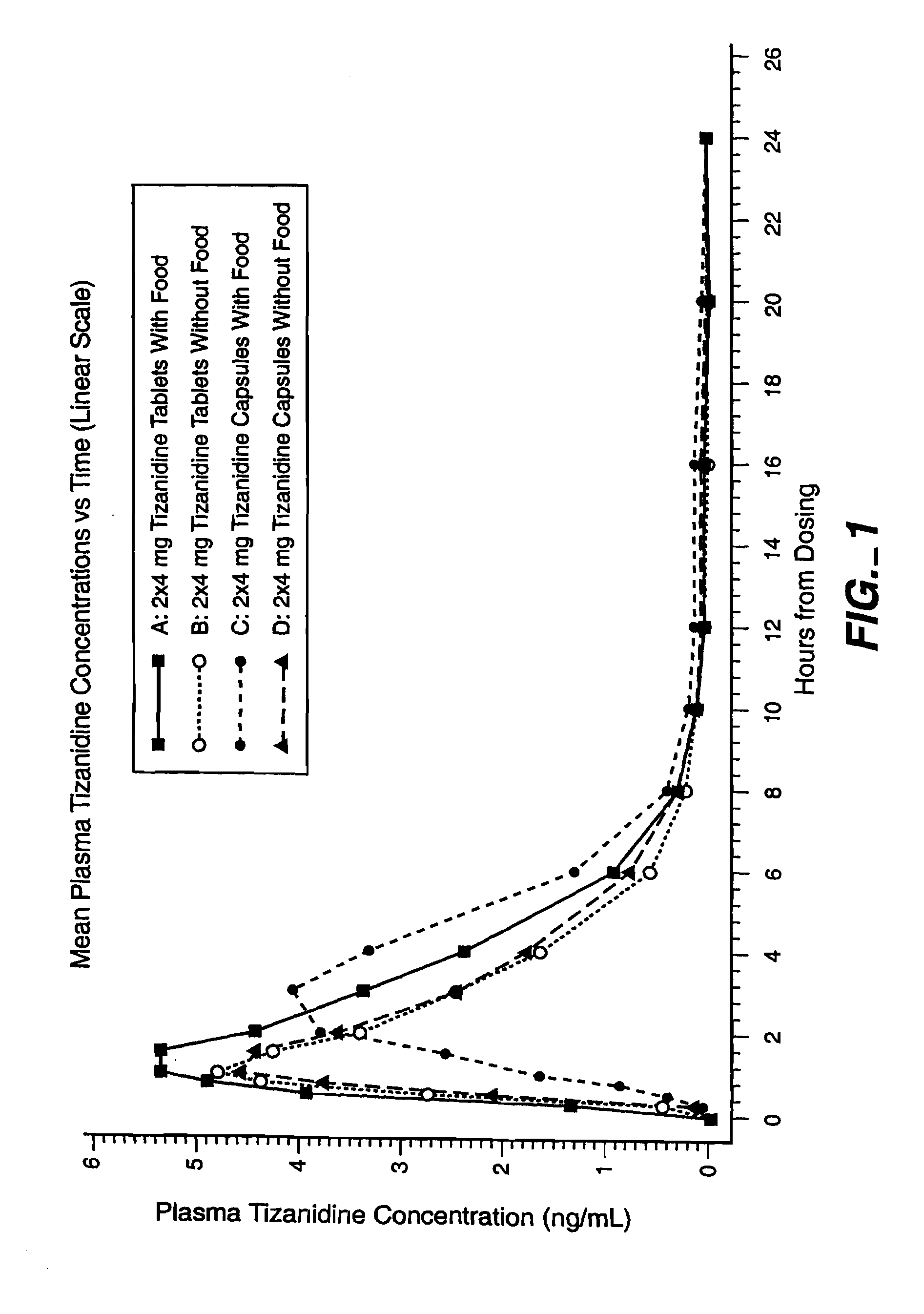
Method of reducing somnolence in patients treated with tizanidine
a technology of tizanidine and somnolence, applied in the field of somnolence, can solve the problems of increasing the likelihood of somnolence, and achieve the effect of reducing somnolen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
(a) Immediate Release Multiparticulates
[0060]A Tizanidine HCl Application Solution is prepared as described in the Description of Individual Process Steps above according to the formulation in Table 3. The Tizanidine HCl Application Solution is then coated onto nonpareil sseds to a level of approximately 7.0% solids weight gain using for example a Glatt GPCG 5 (Glatt, Protech Ltd., Leicester, UK) fluid bed coating apparatus to form Immediate Release Multiparticulates as described in the Description of Individual Process Steps above.
TABLE 3Tizanidine HCl Application SolutionAmountIngredient(% w / w)Tizanidine HCl3.59Hydroxypropyl Methylcellulose 6 cps2.50Silicon Dioxide1.65Purified Water92.26
[0061]Immediate Release Capsules
[0062]The Immediate Release Multiparticulates prepared according to Example 2(a) above are encapsulated into hard gelatin capsules to the required dosage strength as described in the Description of Individual Process Steps above.
TABLE 4Immediate Release Capsules2 mg ...
example 3
(a) Immediate Release Multiparticulates
[0064]A Tizanidine HCl Application Solution is prepared as described in the Description of Individual Process Steps above according to the formulation in Table 5. The Tizanidine HCl Application Solution is then coated onto non-pareil seeds to a level of approximately 9.5% solids weight gain using for example a Glatt GPCG 3 (Glatt, Protech Ltd., Leicester, UK) fluid bed coating apparatus to form Immediate Release Multiparticulates as described in the Description of Individual Process Steps above.
TABLE 5Tizanidine HCl Application SolutionAmountIngredient(% w / w)Tizanidine HCl3.59Polyvinylpyrrolidone4.96Silicon Dioxide1.65Purified Water89.79
(b) Immediate Release Capsules
[0065]The Immediate Release Multiparticulates prepared according to Example 3(a) above are encapsulated into hard gelatin capsules to the required dosage strength as described in the Description of Individual Process Steps above.
TABLE 6Immediate Release Capsules2 mg Capsule4 mg Caps...
example 4
(a) Immediate Release Multiparticulates
[0067]A Tizanidine HCl Application Solution is prepared as described in the Description of Individual Process Steps above according to the formulation in Table 7. The Tizanidine HCl Application Solution is then coated onto non-pareil seeds to a level of approximately 8.6% solids weight gain using for example a Glatt GPCG 30 (Glatt, Protech Ltd., Leicester, UK) fluid bed coating apparatus to form Immediate Release Multiparticulates as described in the Description of Individual Process Steps above.
TABLE 7Tizanidine HCl Application SolutionAmountIngredient(% w / w)Tizanidine HCl2.54Hydroxypropyl Methylcellulose 3 cps3.95Talc1.50Purified Water91.56
(b) Immediate Release Capsules
[0068]The Immediate Release Multiparticulates prepared according to Example 4(a) above are encapsulated into hard gelatin capsules to the required dosage strength as described in the Description of Individual Process Steps above.
TABLE 8Immediate Release Capsules4 mg Capsule6 mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| diastolic blood pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com