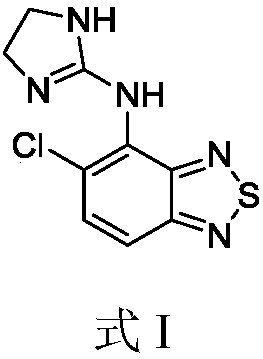
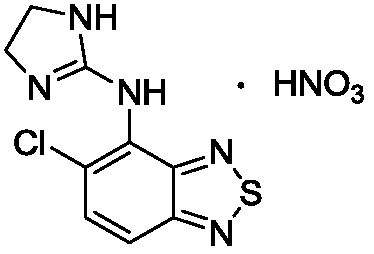
Tizanidine nitrate crystal form A, and preparation method and applications thereof
A tizanidine and crystal form technology, applied in the field of medicinal chemistry, can solve the problems affecting the stability of pharmaceutical preparations, solubility, hygroscopicity, bioavailability, drug quality and clinical efficacy differences, etc. The effect of good performance and good high temperature stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 tizanidine nitrate crystal form A
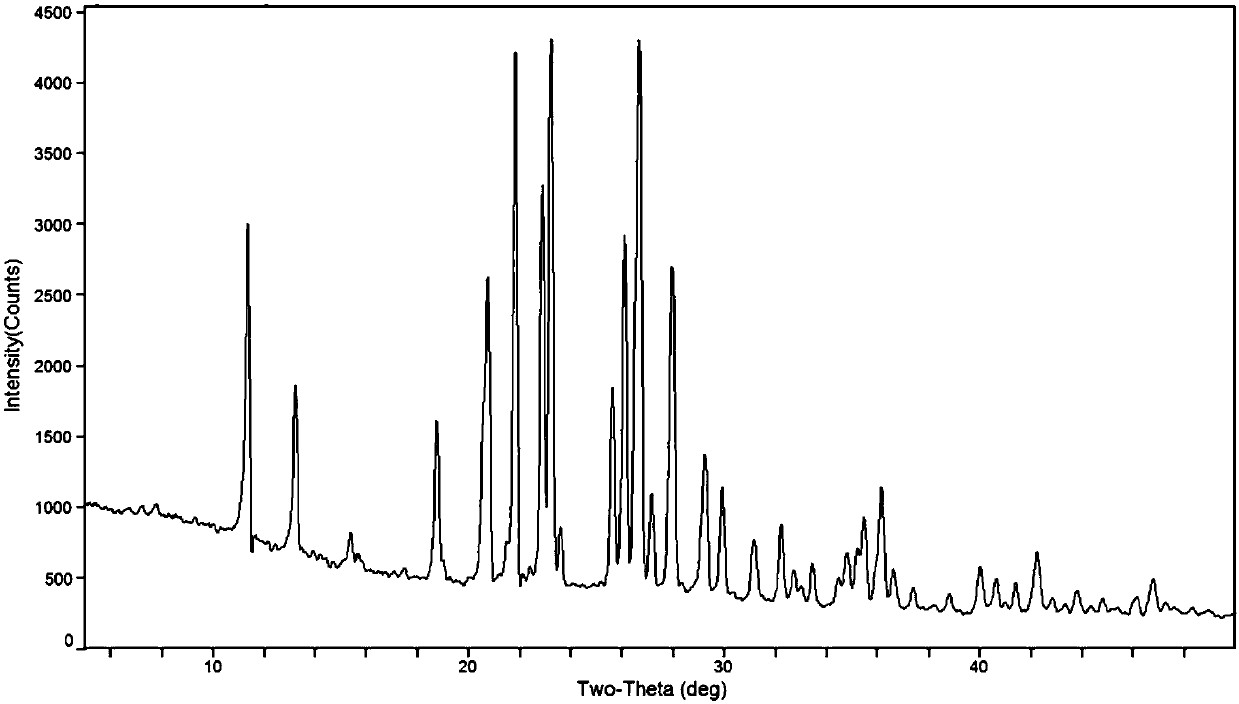
[0036] Weigh 10g of tizanidine, add 400mL of methanol and 2.53mL of concentrated nitric acid, heat up to 40-45°C and stir to react. After the reaction is complete, stop heating, slowly cool down to 5-10°C, and continue to stir and crystallize for 4 hours. After filtration, washing, and vacuum drying at 35° C., 12.07 g of crystal form A of tizanidine nitrate was obtained as a white powder, with a yield of 96.7% and an HPLC purity of 99.98%. Mass spectrum shows its MS (m / z): 253 (M + ).
[0037] Adopt DX-2700 type X-ray powder diffractometer to analyze sample crystal phase, Cu-Kα radiation, tube voltage 35KV, tube current 30mA, record the X-ray powder diffraction pattern of tizanidine nitrate crystal form A see figure 1, and the diffraction related data are shown in Table 1. Among them, the 2θ measurement error is ±0.2°, and the relative intensity measurement error is ±5%, sometimes even up to ±20%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com