Method for preparing dexmedetomidine hydroch
A technology for dexmedetomidine hydrochloride and racemic beauty, which is applied in the field of pharmaceutical preparation, can solve the problems of unspecified chemical purity of split substances, inability to obtain purity, high dexmedetomidine and the like, and achieves the advantages of being beneficial to industrial production, The effect of relatively low cost and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
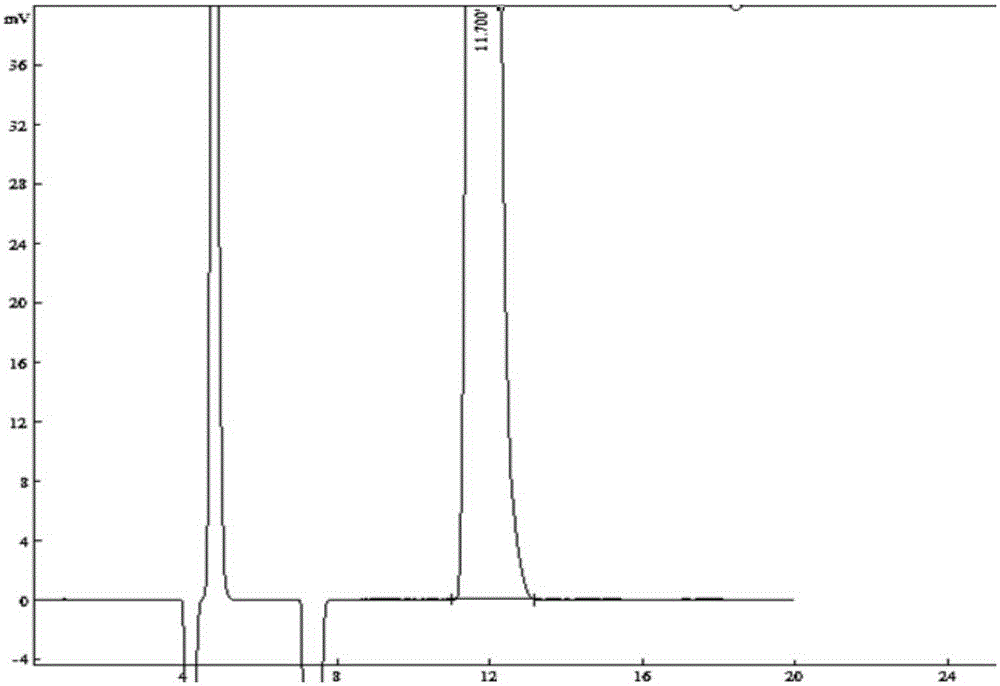
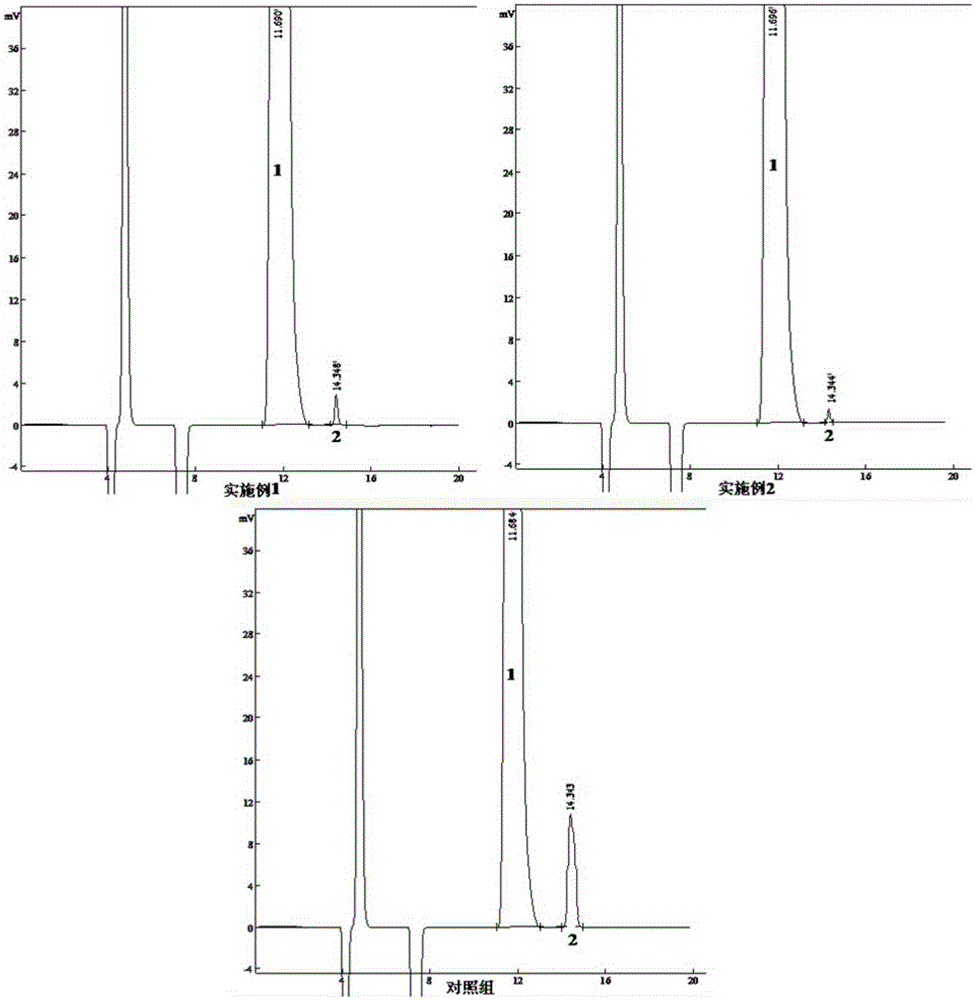
[0017] Preparation of dexmedetomidine: Add 50 g of racemic medetomidine and 18.4 g of S-(+)-mandelic acid into a 500 mL three-necked flask, add 200 mL of methanol and stir for 2 h under heating at 70°C; add 150 mL of diethyl ether Cool and stand for 24 hours to crystallize, filter; add 52.8 g of L-(-)-dibenzoyl tartaric acid to the mother liquor, stir and crystallize for 24 hours, filter to obtain dexmedetomidine, ee% 99.54%.
[0018] The preparation of dexmedetomidine hydrochloride: join dexmedetomidine (36g, 180mmol) and 100mL absolute ethanol in the 1000mL reaction flask, add the 1LHCl / diethyl ether saturated solution that has configured in the system, this moment pH Value is 3, stirred and crystallized, filtered, and the filter cake was recrystallized through ethanol:ether (20ml:200ml), and vacuum-dried to obtain dexmedetomidine hydrochloride (38.0g, yield 90.0%), mp156.0~156.9°C, (Literature value: mp156.5~157.5°C); purity: 99.8%; ee%: 100%.
Embodiment 2
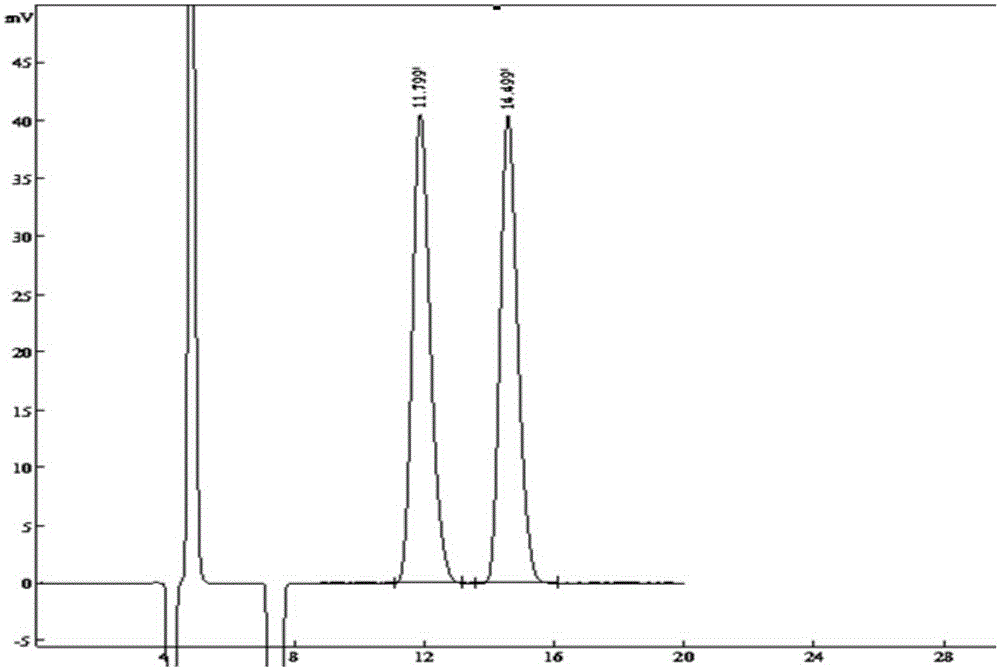
[0020] The preparation of dexmedetomidine: add 50g of racemic medetomidine, 18.4g of S-(+)-mandelic acid and 0.2g of pyracline B into a 500mL three-necked flask, add 200mL of methanol and heat at 70°C Stir under the conditions for 2h; add 150mL ether, cool and stand for 24h to crystallize, filter; add 52.8 grams of L-(-)-dibenzoyl tartaric acid to the mother liquor, stir and crystallize for 24 hours, filter to obtain dexmedetomidine, ee %99.94%.
[0021] The preparation of dexmedetomidine hydrochloride: join dexmedetomidine (36g, 180mmol) and 100mL absolute ethanol in the 1000mL reaction flask, add the 1LHCl / diethyl ether saturated solution that has configured in the system, this moment pH Value is 3, stirred and crystallized, filtered, and the filter cake was recrystallized through ethanol:ether (20ml:200ml), vacuum-dried to obtain dexmedetomidine hydrochloride (38.9g, yield 92.2%), mp156.1~156.9 ℃, (Literature value: mp156.5~157.5°C); purity: 99.9%; ee%: 100%.
[0022] con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com