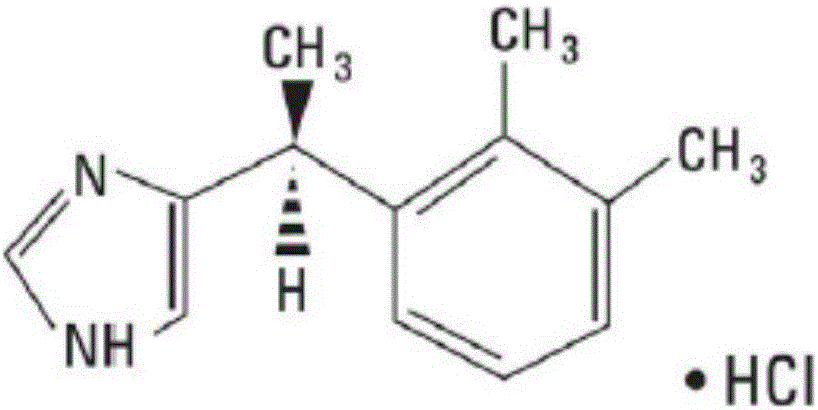
Premixed preparation for dexmedetomidine
A dexmedetomidine and premixing technology, applied in the field of ready-to-use premixed preparations, can solve the problems of rising impurities, hidden dangers of infusion safety, easy breakage, etc., so as to avoid pollution and excess, reduce infusion pollution, and be difficult to break. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Selection of ready-to-use premixed dexmedetomidine composition plastic packaging materials
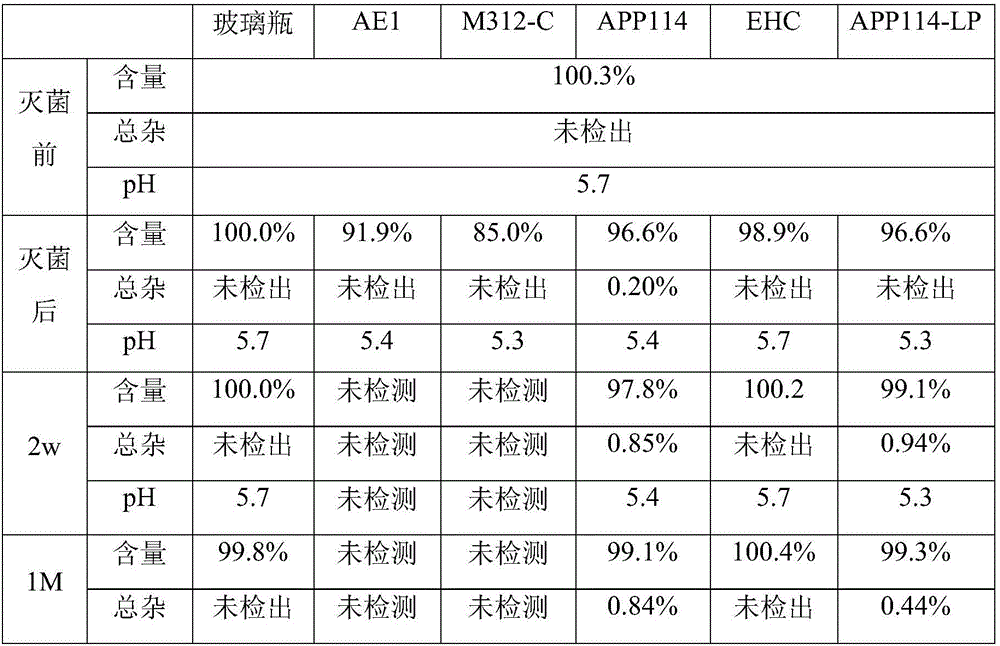
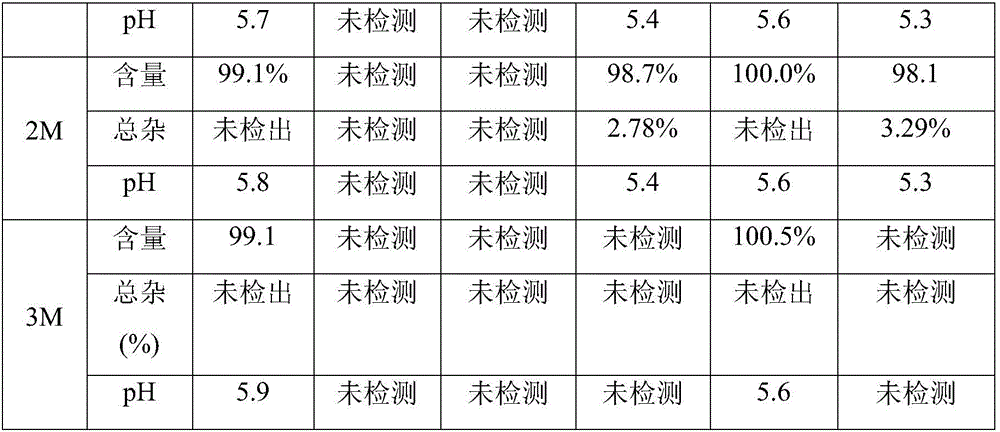
[0029] Dissolve dexmedetomidine hydrochloride and sodium chloride in water for injection, respectively, to prepare a ready-to-use premixed solution containing 4 μg dexmedetomidine and 9 mg sodium chloride per milliliter, and filter through a 0.22 μ PVDF filter membrane Afterwards, 50 mL of the inner layer is divided into multi-layer co-extruded infusion soft bags made of different materials, and sterilized at 121 degrees for 15 minutes. The sterilized samples were placed under accelerated conditions (40°C / 75%RH) for stability investigation. The multi-layer co-extruded infusion soft bags with different inner layers are made by heat-sealing the infusion films from different manufacturers, including German Polysini APP114 (inner layer is polypropylene), German Polysini APP114-LP (inner The layer is polypropylene), Sealed Air's M312-C (modified ethylene / propylene polymer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com