Method for preparing dexmedetomidine and hydrochloride thereof
A hydrochloride and catalyst technology, which is applied in the field of preparation of dexmedetomidine and its hydrochloride, can solve the problems of long time consumption, unfavorable production, waste of left-handed products, etc., and achieve high yield without chiral disassembly The effect of short synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The preparation method of formula I compound
[0077] Provide a kind of preparation method of formula I compound, described method comprises steps:
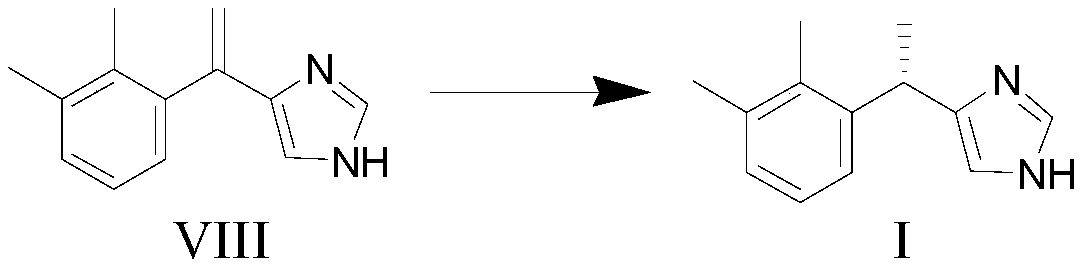
[0078] (5) In the fifth solvent, in the presence of a catalyst and a hydrogen source, the compound of formula VIII undergoes a hydrogenation reduction reaction to obtain a compound of formula I,
[0079]
[0080] Wherein, the catalyst includes a carbon-carbon hydrogenation reduction catalyst and (R,S)-Duanphos.
[0081] In another preferred example, the total weight of the carbon-carbon hydrogenation reduction catalyst and (R,S)-Duanphos accounts for 50-100% of the total weight of the catalyst, preferably 80-100%, more preferably , 90-100%.
[0082] In another preference, the carbon-carbon hydrogenation reduction catalyst is selected from: Rh(COD) 2 BF 4 , palladium on carbon, sodium hydroxide palladium on carbon, platinum on carbon, palladium acetate, or combinations thereof.
[0083] In another preferred embodime...
Embodiment 1
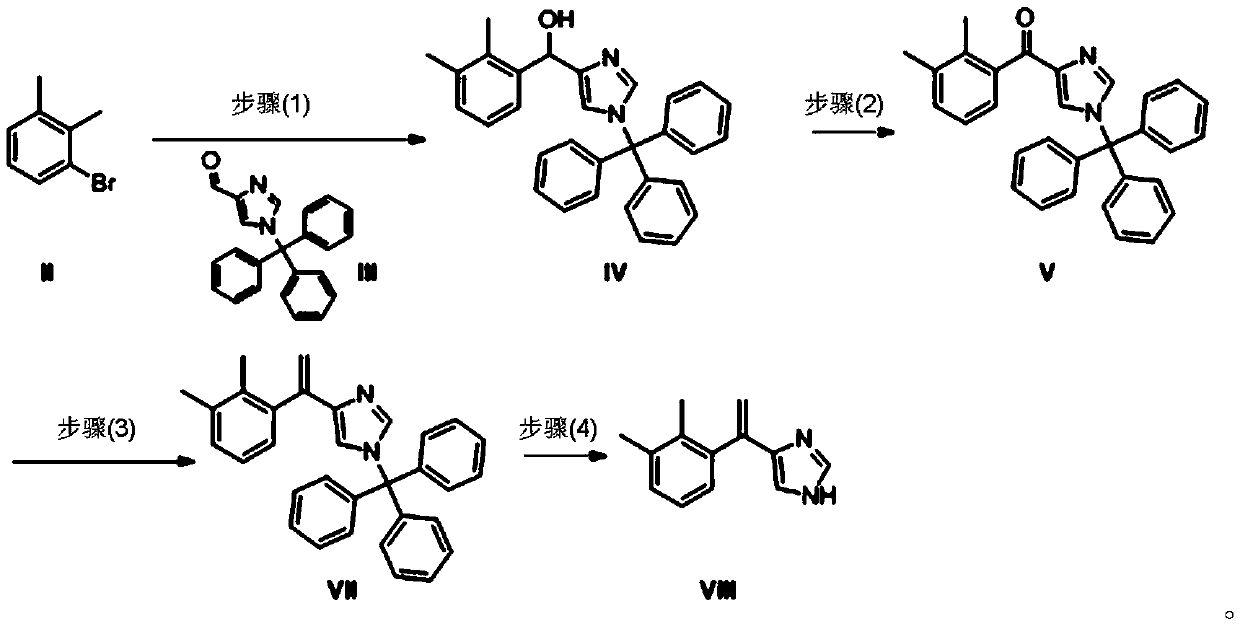
[0110] Dissolve the compound of formula II (164.0 g, 0.67 mol) in THF (720 mL) and set aside;
[0111] Add magnesium chips (26g, 1.08mol) and THF (240mL) to a 2L three-necked reaction flask under nitrogen protection, add 1 grain of elemental iodine, add the tetrahydrofuran solution (10mL) of the compound of formula II at one time, and heat up to reflux (internal temperature = 70°C) , add dropwise the tetrahydrofuran solution of the remaining compound of formula II, keep reflux for 1.5h, and cool to room temperature for later use.
[0112]The compound of formula III (120g, 0.35mol,) was dissolved in tetrahydrofuran (1.2L), under nitrogen protection, cooled to -5°C, added dropwise the newly prepared Grignard reagent, controlled at -5-0°C, after the addition was completed, raised to room temperature After the reaction was completed, saturated ammonium chloride (700mL) was added dropwise, and DCM (2.5L) was added for extraction. The organic phase was washed with saturated brine (1...
Embodiment 2
[0114] Formula IV compound (50g, 0.11mol) was added in 1,4-dioxane (750mL), MnO 2 (100g, 1.15mol), heated to reflux, reacted for 3h, the reaction was complete, filtered while it was hot, and the filtrate was concentrated to dryness to obtain a white solid crude product, which was added to methanol (350mL) and beaten at room temperature (25°C) for 1-2h, filtered, and dried in vacuo to obtain White solid compound of formula V (45 g), yield: 90.0%. MS (ESI): [M+1] + = 443.20.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com