Pharmaceutical product comprising transnasal dexmedetomidine composition
A technology of dexmedetomidine and a composition, applied in the field of pharmaceutical preparations, can solve problems such as product deterioration, increase in foreign bodies, deepening color, etc., and achieve the effects of satisfying clinical sedation, excellent stability, and avoiding inflammation problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
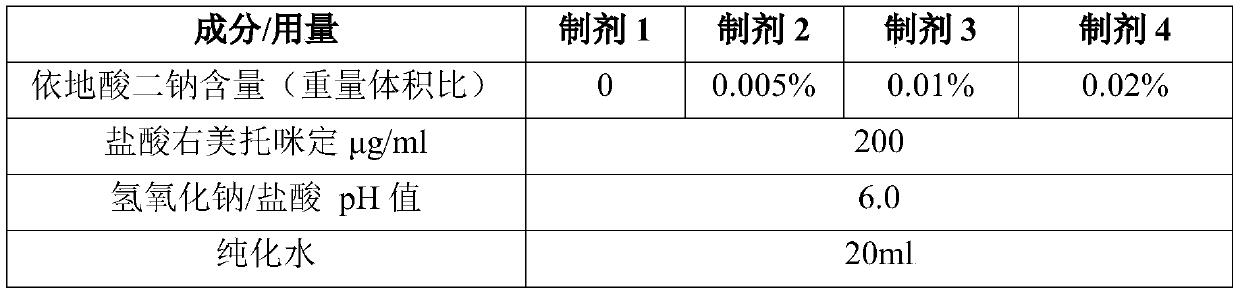
[0038] Preparation prescription:
[0039]
[0040] Use a stainless steel container to prepare, weigh the prescribed amount of edetate disodium, add about 70% of the total volume to purified water, and stir to dissolve. Add the prescribed amount of dexmedetomidine hydrochloride to the above solution, and stir to dissolve. Adjust the pH value of the solution to 6.0 with sodium hydroxide, and add purified water to the full amount. After contacting with stainless steel for 4 hours, the liquid medicine is divided into 5ml medium borosilicate glass molded bottles, and the cap is screwed on by the spray pump.
Embodiment 2
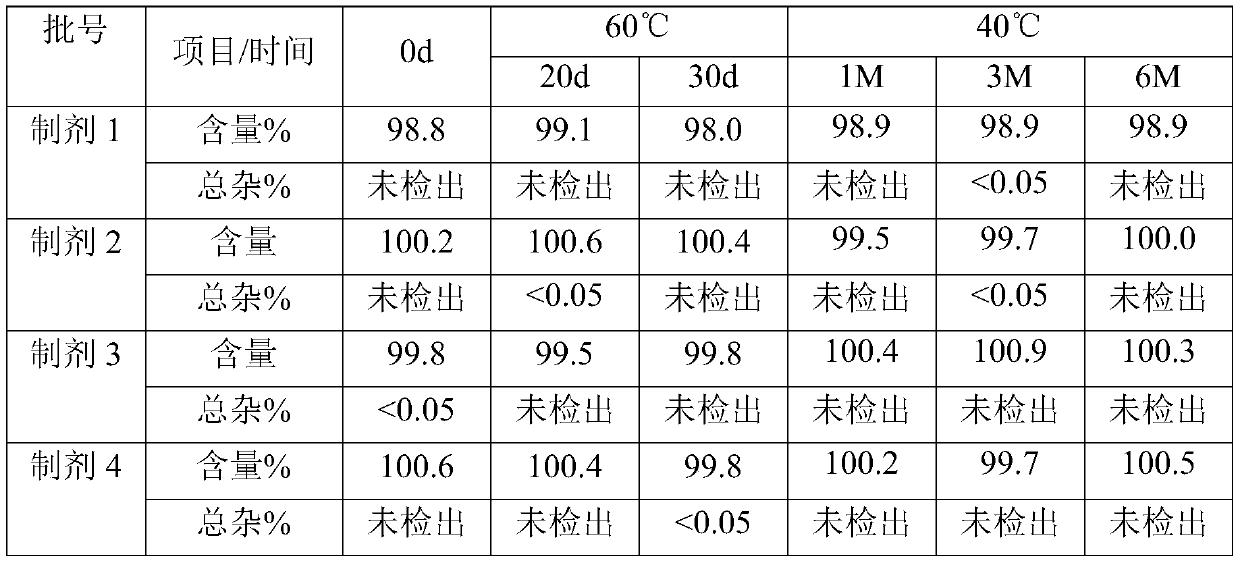
[0042]Each preparation product of Example 1 was placed in a constant temperature and humidity chamber at 40°C and 60°C, and the stability of the product was investigated with content, related substances and other items as the main inspection indicators. Detection method: detection by high performance liquid chromatography system, the chromatographic column is Waters X-BridgeTM Shield RP18 (5μm, 4.6×250mm), mobile phase A is 0.1M sodium phosphate buffer solution (add 1% triethylamine, adjust pH to 3.5), mobile phase B is methanol, detection wavelength: 214nm. The test results are shown in the table below.
[0043]
[0044] The results show that there is no significant difference in the stability of the preparation without EDTA-2Na compared with adding 0.005%, 0.01%, 0.02% EDTA-2Na. In addition, after preparation 1 was placed under various conditions, the preparation system was still colorless and clear, with a good appearance.
Embodiment 3
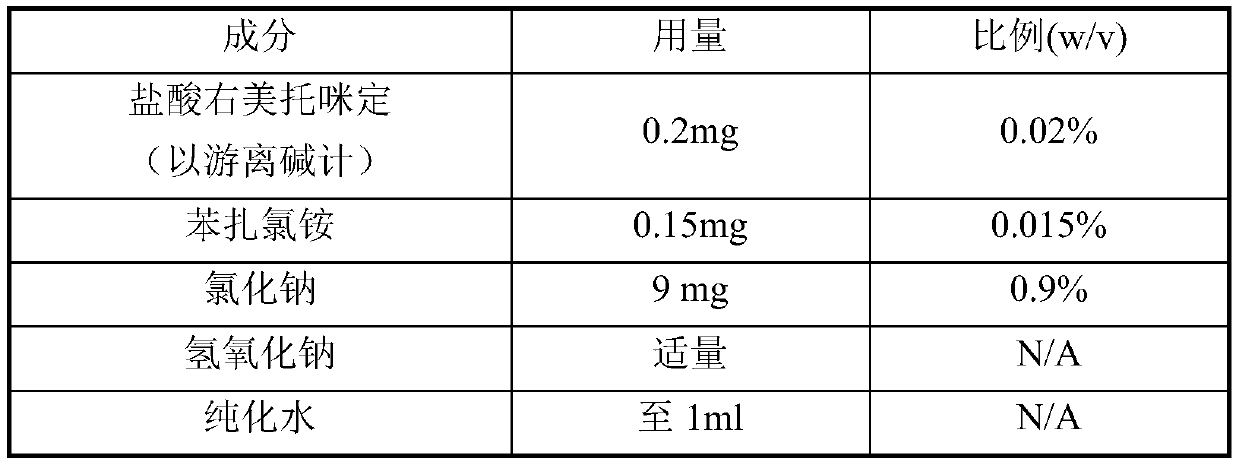
[0046]
[0047] Use a stainless steel container to prepare, weigh the benzalkonium chloride and sodium chloride of the prescription amount, add about 90% of the total volume of the preparation in purified water, stir at room temperature until dissolved, then add the prescription amount of dexmedetomidine hydrochloride, continue to stir until Dissolve, adjust the pH to 6.0 with 0.1M sodium hydroxide, add purified water to the full amount, and continue stirring until the mixture is uniform. Use a peristaltic pump to filter the prepared drug solution through a 0.45 μm filter disc (PES), and finally fill it into a 5 ml medium borosilicate glass molded bottle, and screw the cap with a spray pump.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com