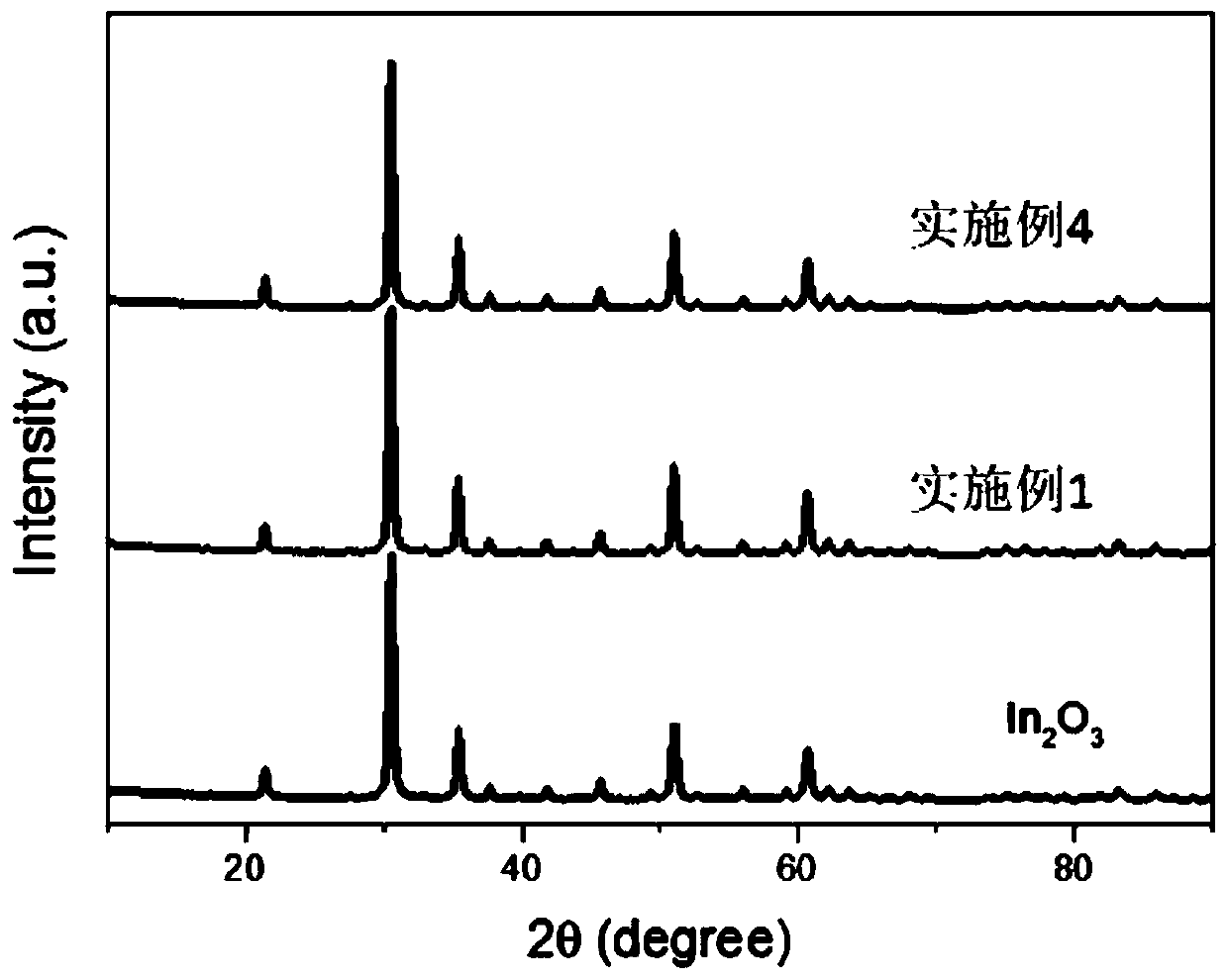
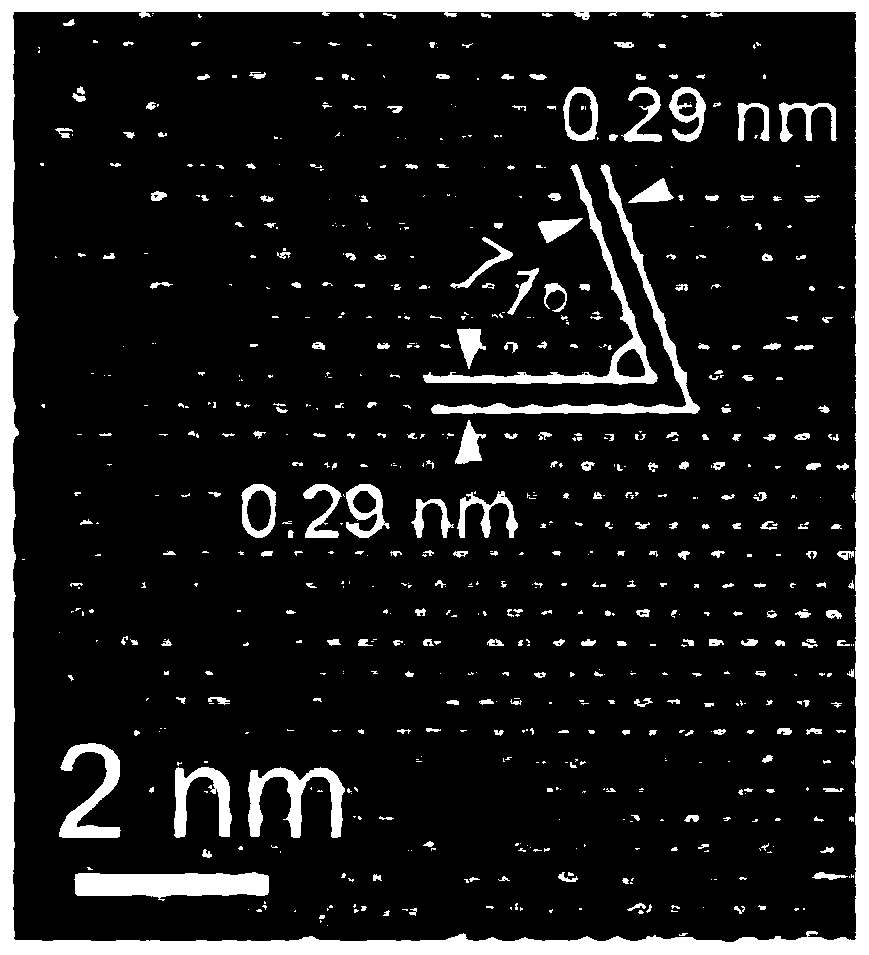
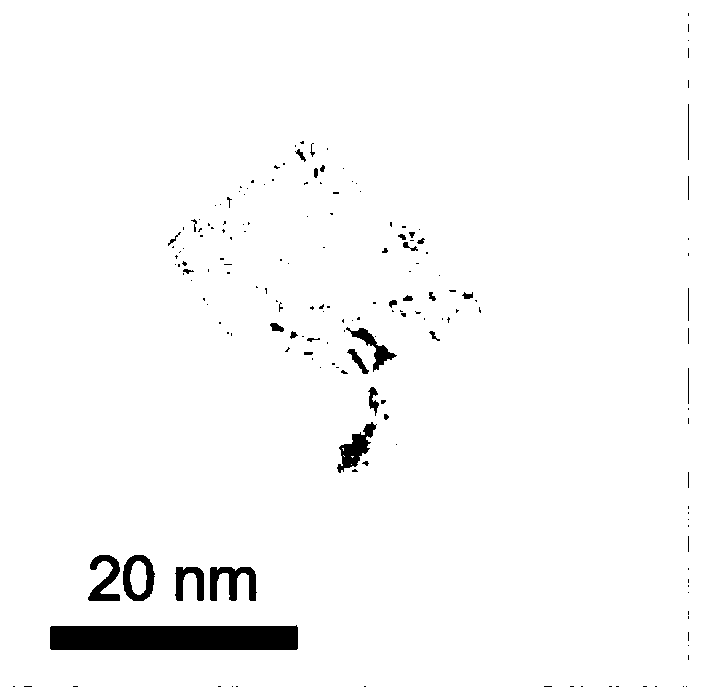
Method for electrochemically extracting uranium from seawater by using oxygen vacancy-containing metal oxide
An oxide and oxygen vacancy technology, applied in chemical instruments and methods, uranium oxide/hydroxide, uranium dioxide, etc., can solve the problems of difficult recycling, poor selectivity, and high production costs, and achieve a simple extraction method. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for electrochemically extracting uranium from seawater using metal oxides containing oxygen vacancies, comprising the following steps:
[0035] Step 1, add 3g In(NO 3 ) 3 4.5H 2 O was dissolved in 300mL of isopropanol, stirred for 0.5 hours, and ultrasonicated for 1 hour to obtain an indium nitrate isopropanol solution; 100 g of glycerol was added to the indium nitrate in isopropanol solution, stirred for 0.5 hours, and ultrasonicated for 0.5 hours to obtain a mixed solution ; The power of the ultrasound is 800W, and the frequency is 35KHz;
[0036] Step 2. Transfer the mixed solution to a polytetrafluoroethylene high-temperature and high-pressure reactor, raise the temperature to 180°C at a rate of 5°C / min, keep it warm for 1 hour, and cool to room temperature naturally, then separate the solid from the liquid, and use deionized water to separate the solids. Wash with ethanol, then dry in a vacuum oven at 60°C for 12 hours to obtain a spherical indium hydro...
Embodiment 2
[0043] A method for electrochemically extracting uranium from seawater using metal oxides containing oxygen vacancies, comprising the following steps:
[0044] Step 1, add 3g In(NO 3 ) 3 4.5H 2 O was dissolved in 300mL isopropanol, stirred for 1 hour, and ultrasonicated for 0.5 hours to obtain an indium nitrate isopropanol solution; 100 g of glycerol was added to the indium nitrate isopropanol solution, stirred for 1 hour, and ultrasonically used for 0.5 hours to obtain a mixed solution ; The power of the ultrasound is 800W, and the frequency is 40KHz;
[0045] Step 2. Transfer the mixed solution to a polytetrafluoroethylene high-temperature and high-pressure reactor, raise the temperature to 185°C at a rate of 5°C / min, keep it warm for 2 hours, and cool it to room temperature naturally, then separate the solid from the liquid, and use deionized water to separate the solids. Wash with ethanol, then dry in a vacuum oven at 60°C for 12 hours to obtain a spherical indium hydro...
Embodiment 3
[0052] A method for electrochemically extracting uranium from seawater using metal oxides containing oxygen vacancies, comprising the following steps:
[0053] Step 1, add 3g In(NO 3 ) 3 4.5H 2 O was dissolved in 300mL of isopropanol, stirred for 1 hour, and ultrasonicated for 1 hour to obtain an indium nitrate isopropanol solution; 100 g of glycerol was added to the indium nitrate in isopropanol solution, stirred for 1 hour, and ultrasonicated for 1 hour to obtain a mixed solution ; The power of the ultrasound is 800W, and the frequency is 40KHz;
[0054] Step 2. Transfer the mixed solution to a polytetrafluoroethylene high-temperature and high-pressure reactor, raise the temperature to 200°C at a rate of 5°C / min, and keep it warm for 1 hour. After cooling to room temperature naturally, separate the solid and liquid, and use deionized water to separate the solids. Wash with ethanol, then dry in a vacuum oven at 60°C for 12 hours to obtain a spherical indium hydroxide solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com