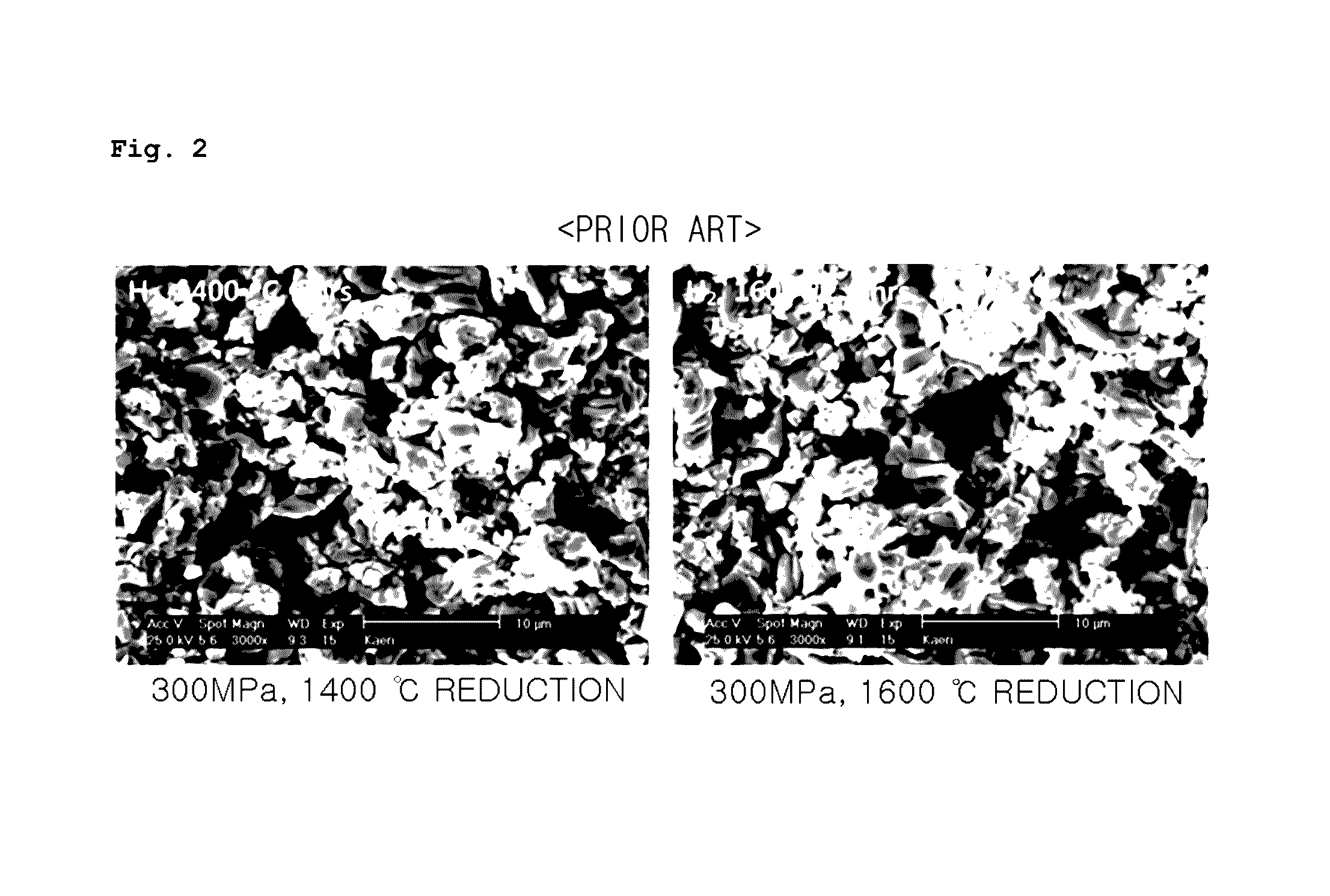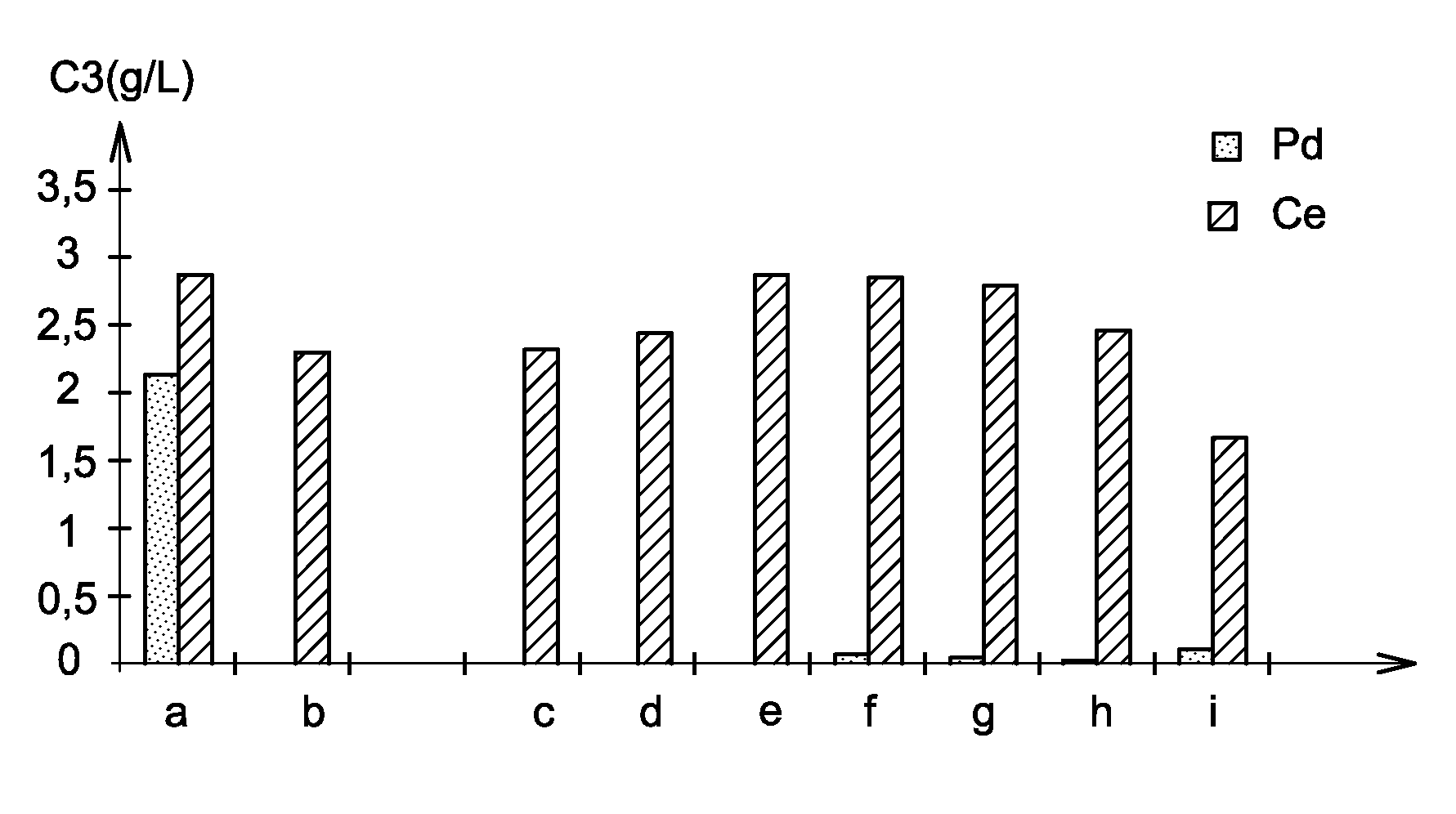Patents
Literature
35results about "Nuclear fuel reprocessing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
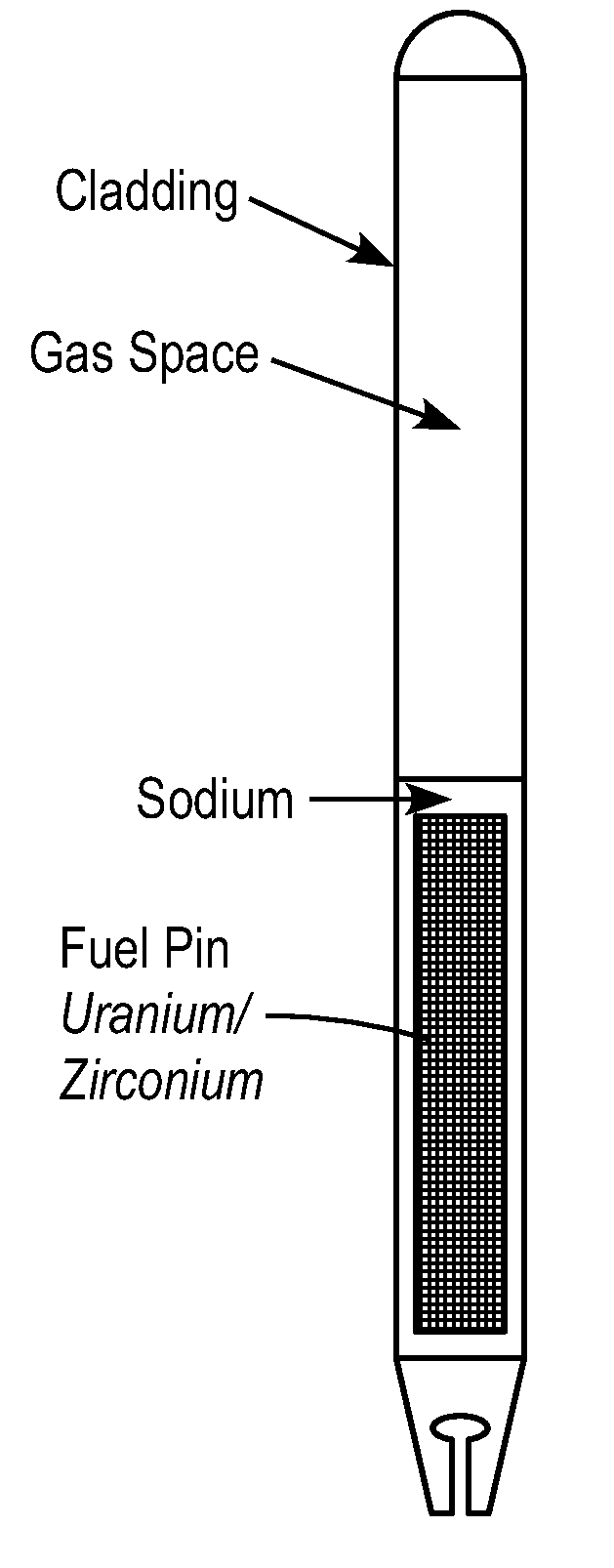
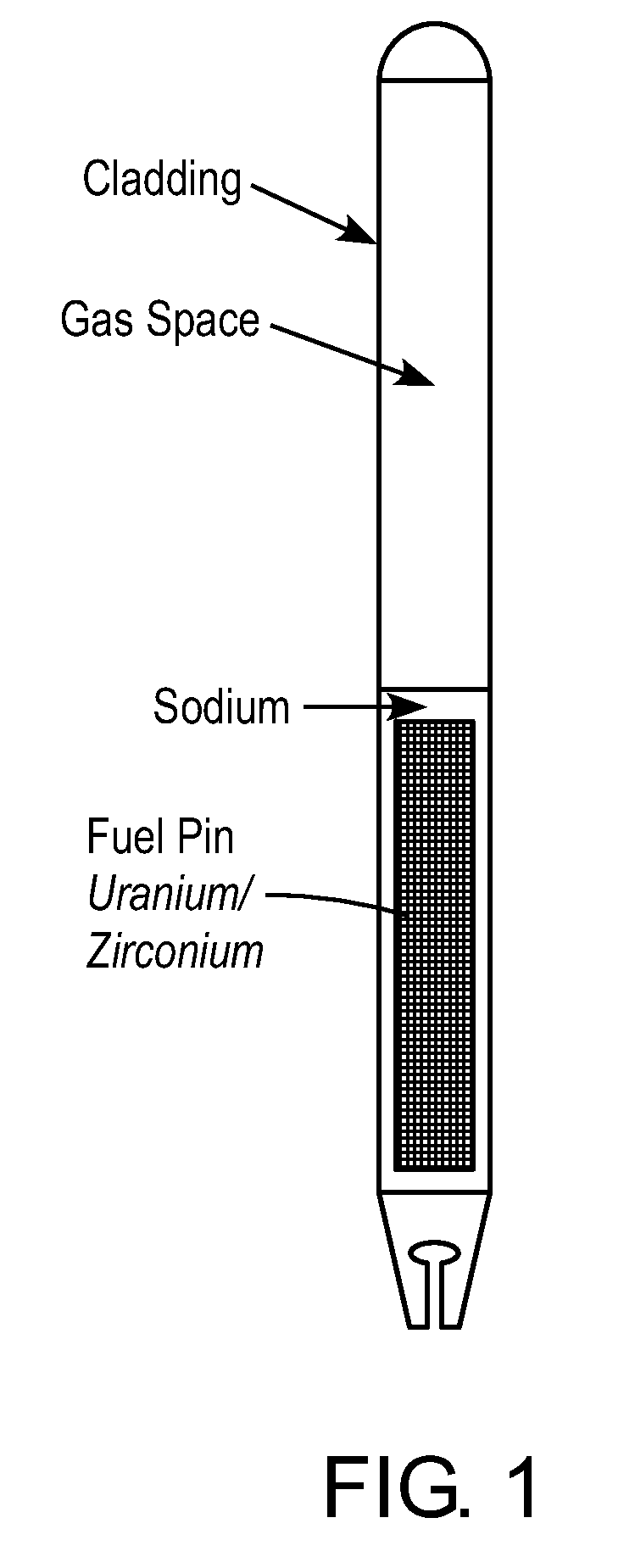
Particulate metal fuels used in power generation, recycling systems, and small modular reactors
ActiveUS20100303193A1Fix fuel costEliminate needNuclear fuel reprocessingFuel elementsNuclear reactor coreNuclear reactor
A metal particulate fuel system is described. The metal fuel system may include particulate metal fuel for use in nuclear reactors. The particulate metal fuel may include a plurality of particles of at least one enriched alloy where the particles are compacted into a fuel column. The metal particulate fuel system may also include a cladding and / or a gas-filled plenum.
Owner:ADVANCED REACTOR CONCEPTS
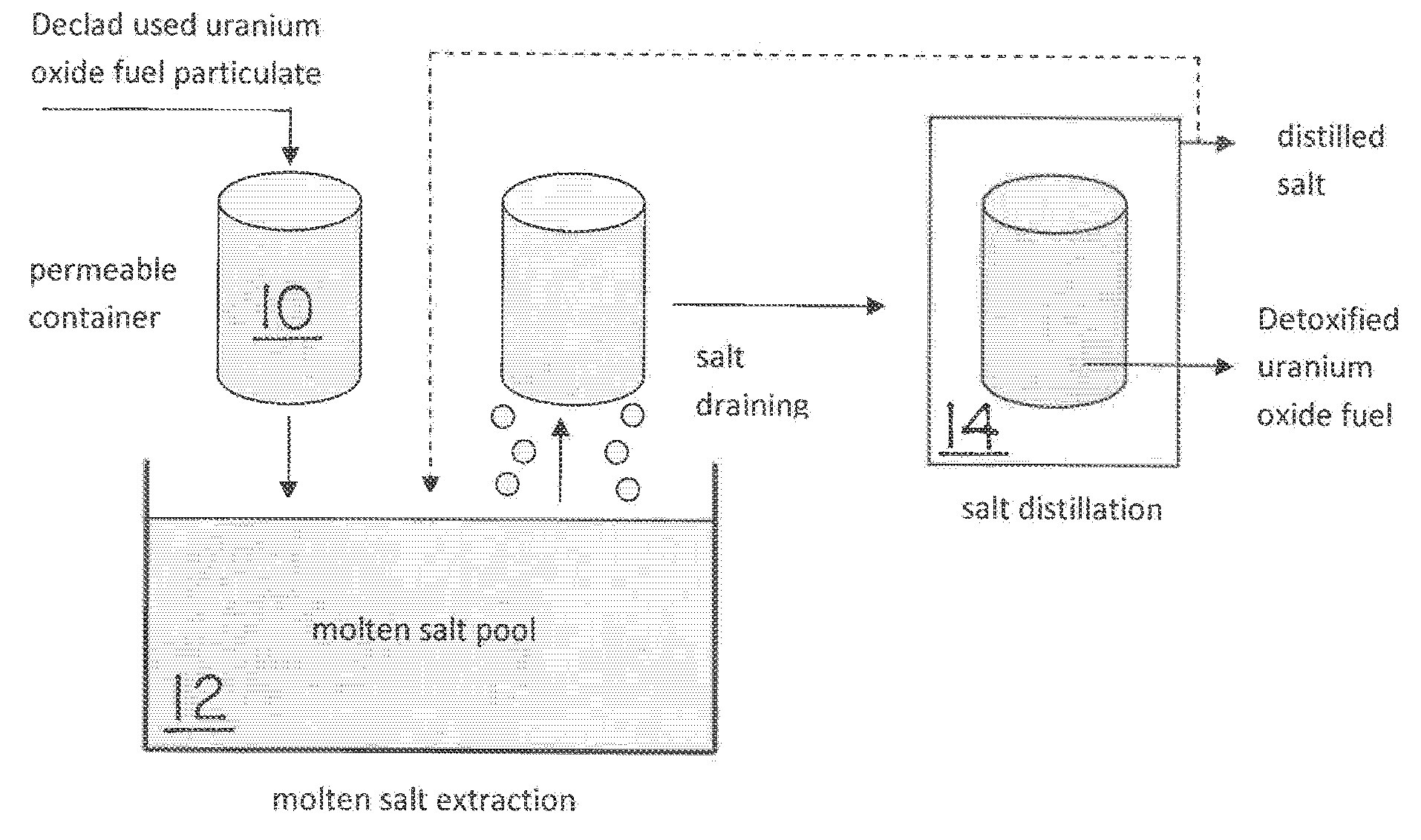
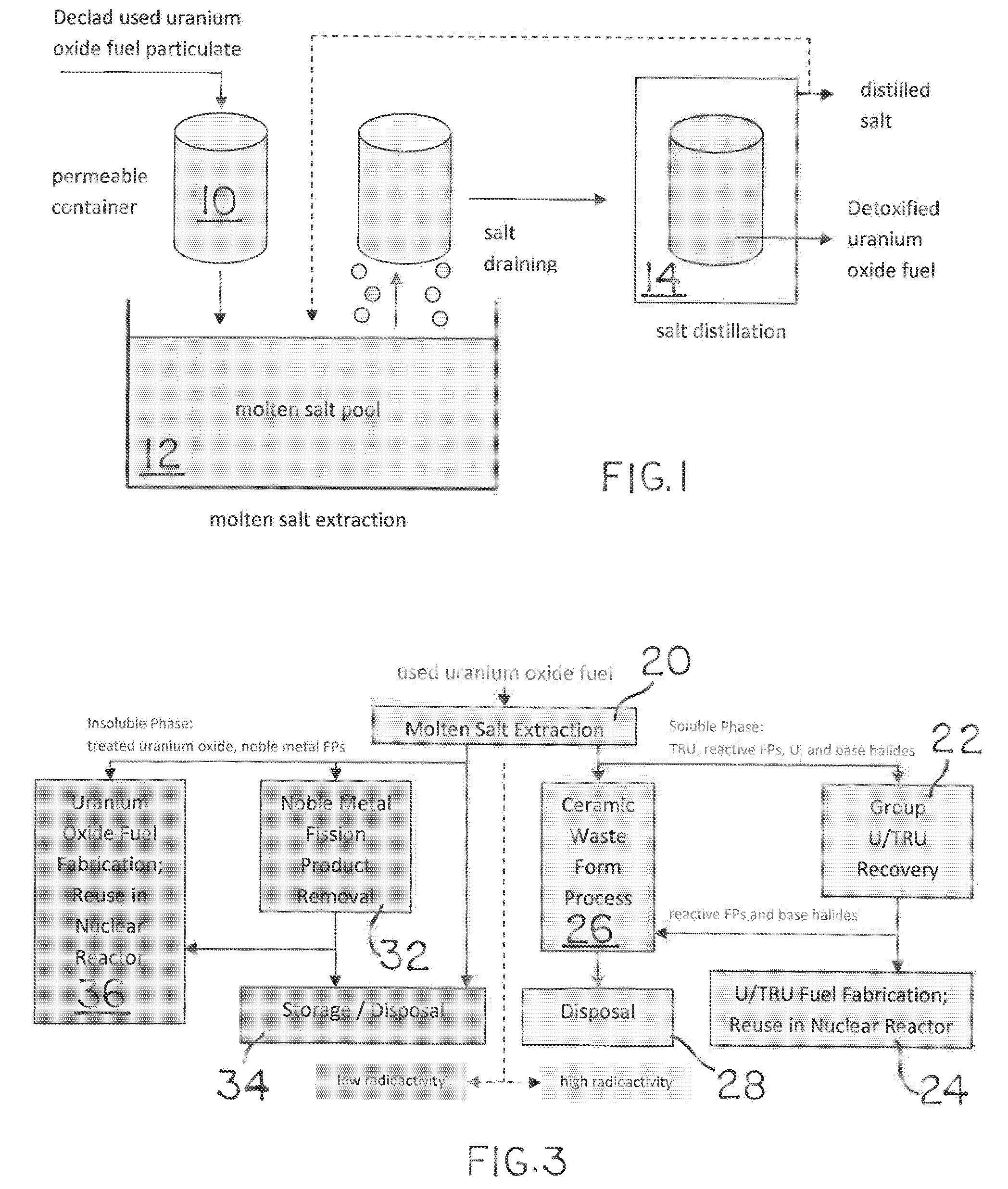
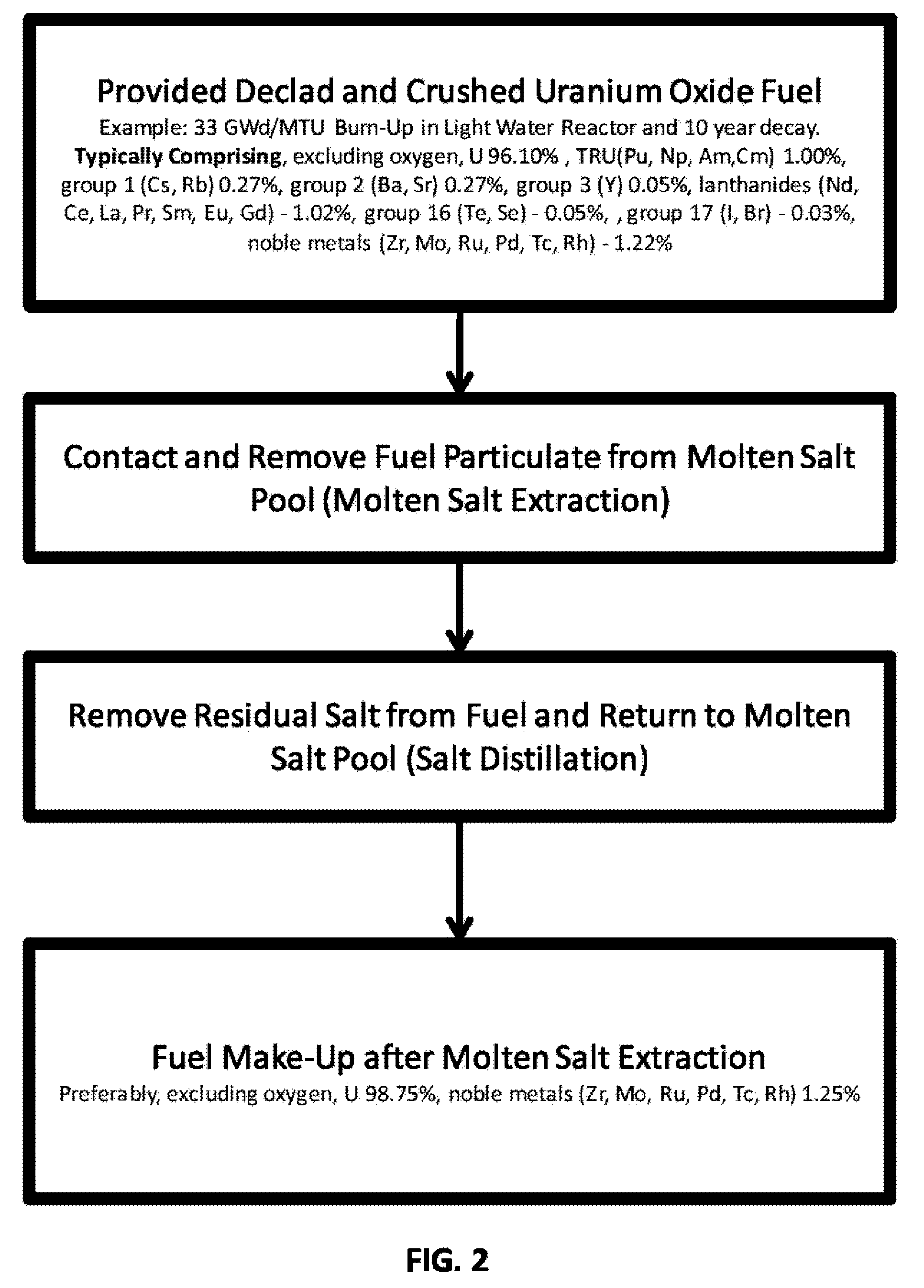
Molten salt extraction of transuranic and reactive fission products from used uranium oxide fuel
Used uranium oxide fuel is detoxified by extracting transuranic and reactive fission products into molten salt. By contacting declad and crushed used uranium oxide fuel with a molten halide salt containing a minor fraction of the respective uranium trihalide, transuranic and reactive fission products partition from the fuel to the molten salt phase, while uranium oxide and non-reactive, or noble metal, fission products remain in an insoluble solid phase. The salt is then separated from the fuel via draining and distillation. By this method, the bulk of the decay heat, fission poisoning capacity, and radiotoxicity are removed from the used fuel. The remaining radioactivity from the noble metal fission products in the detoxified fuel is primarily limited to soft beta emitters. The extracted transuranic and reactive fission products are amenable to existing technologies for group uranium / transuranic product recovery and fission product immobilization in engineered waste forms.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
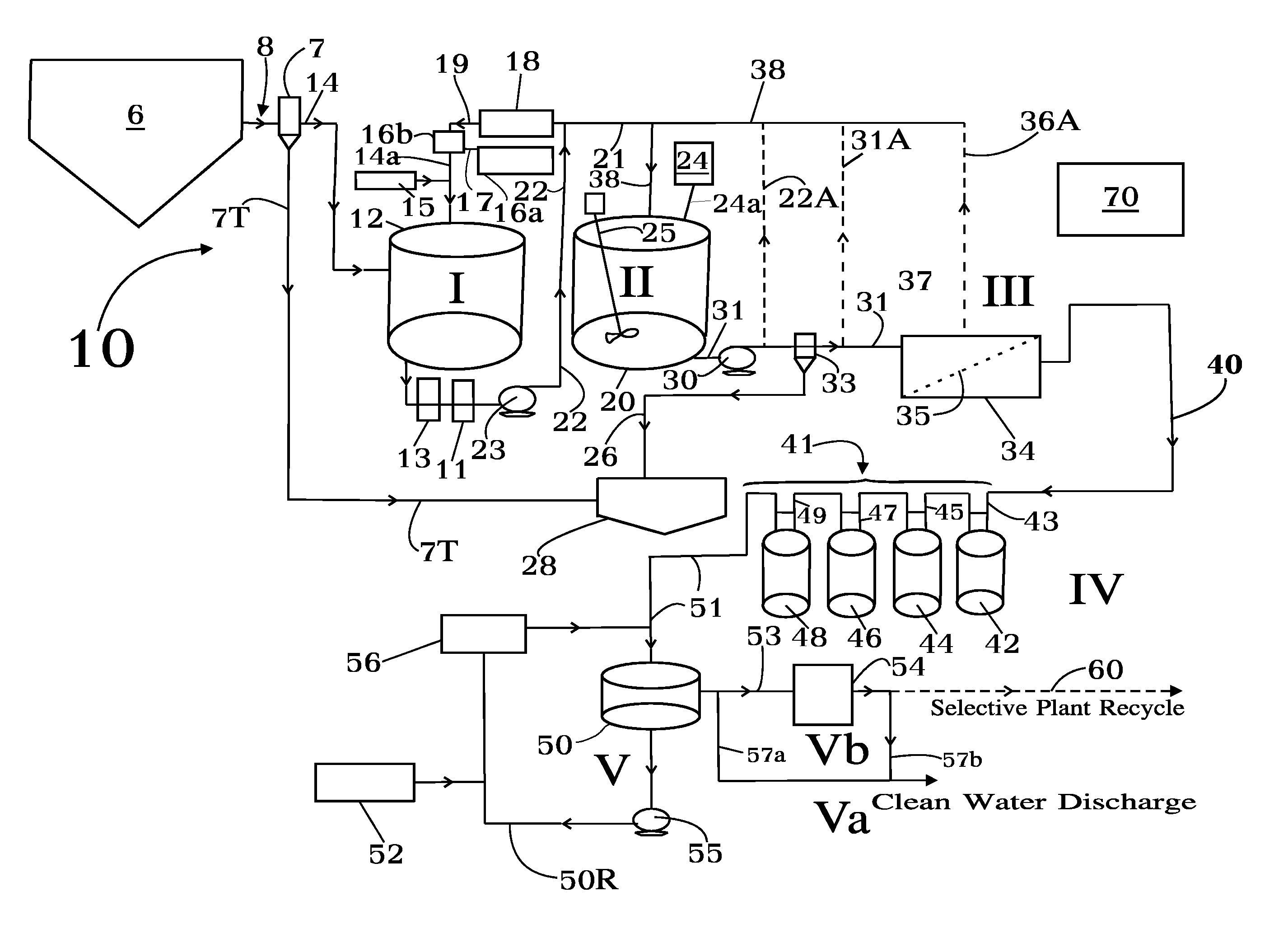
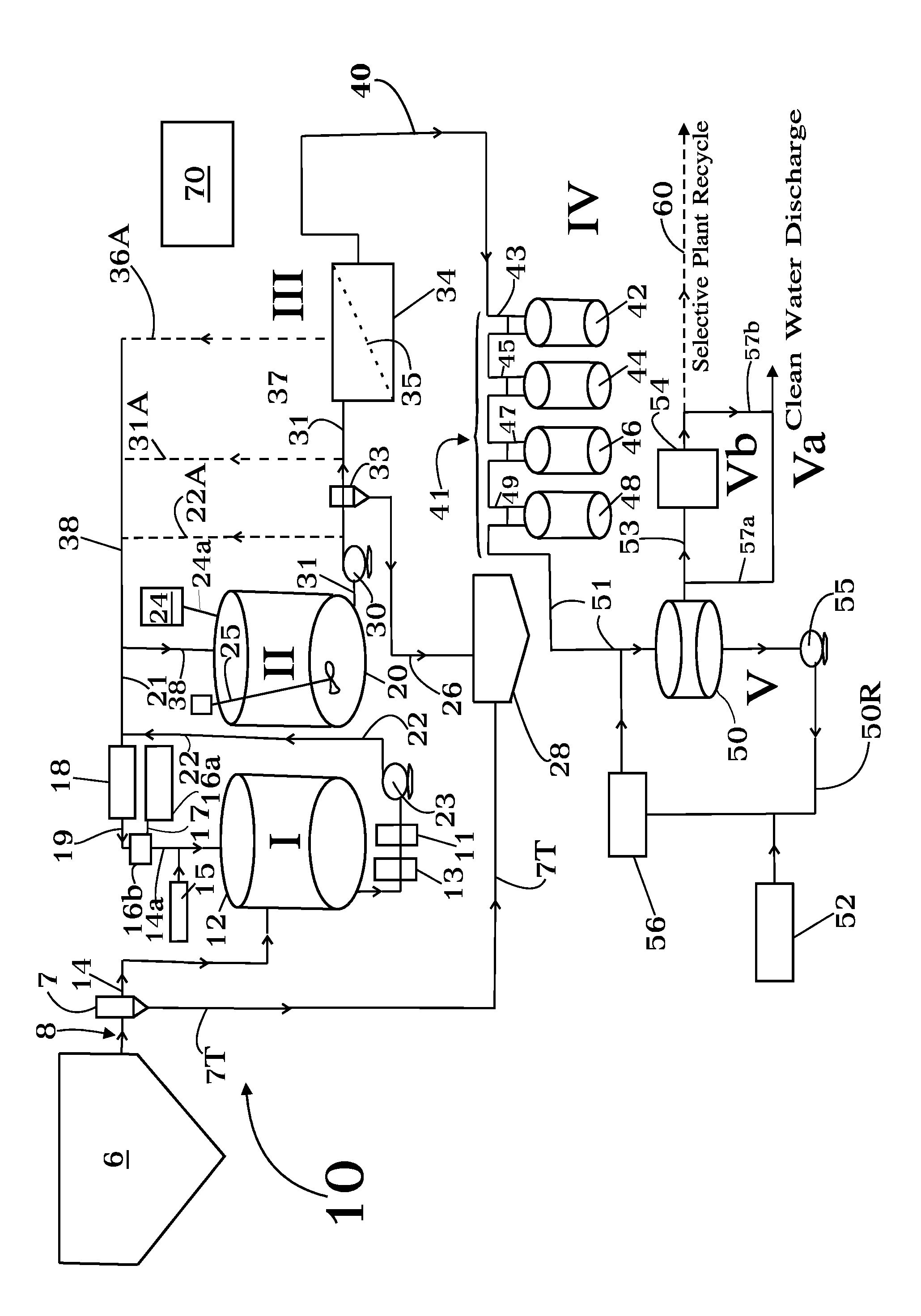
Concentrate treatment system
In one aspect the invention provides a system for treating a wastestream, particularly a radwaste, for safe disposal and, in final processing converting it into one or both forms including an aqueous form for safe discharge to the environment and a solidified form for safe disposal. In another aspect the invention provides the capacity to employ a step where a specific target element strategy can be set up synchronizing sorbent substance choices and multiple recycle options to remove target substances from wastestream as a part of its Sorption or Powder Sorbent Isotopic Reduction step (II). Other steps cooperate with Sorption step (II) including Oxidation (I), Solid-Liquid separation (III), and Selective Ion Exchange (IV) to deliver the wastestream to final processing.
Owner:AVANTECH LLC
Reduction-oxidation of actinides extraction process (ROANEX) for used nuclear fuel recycling
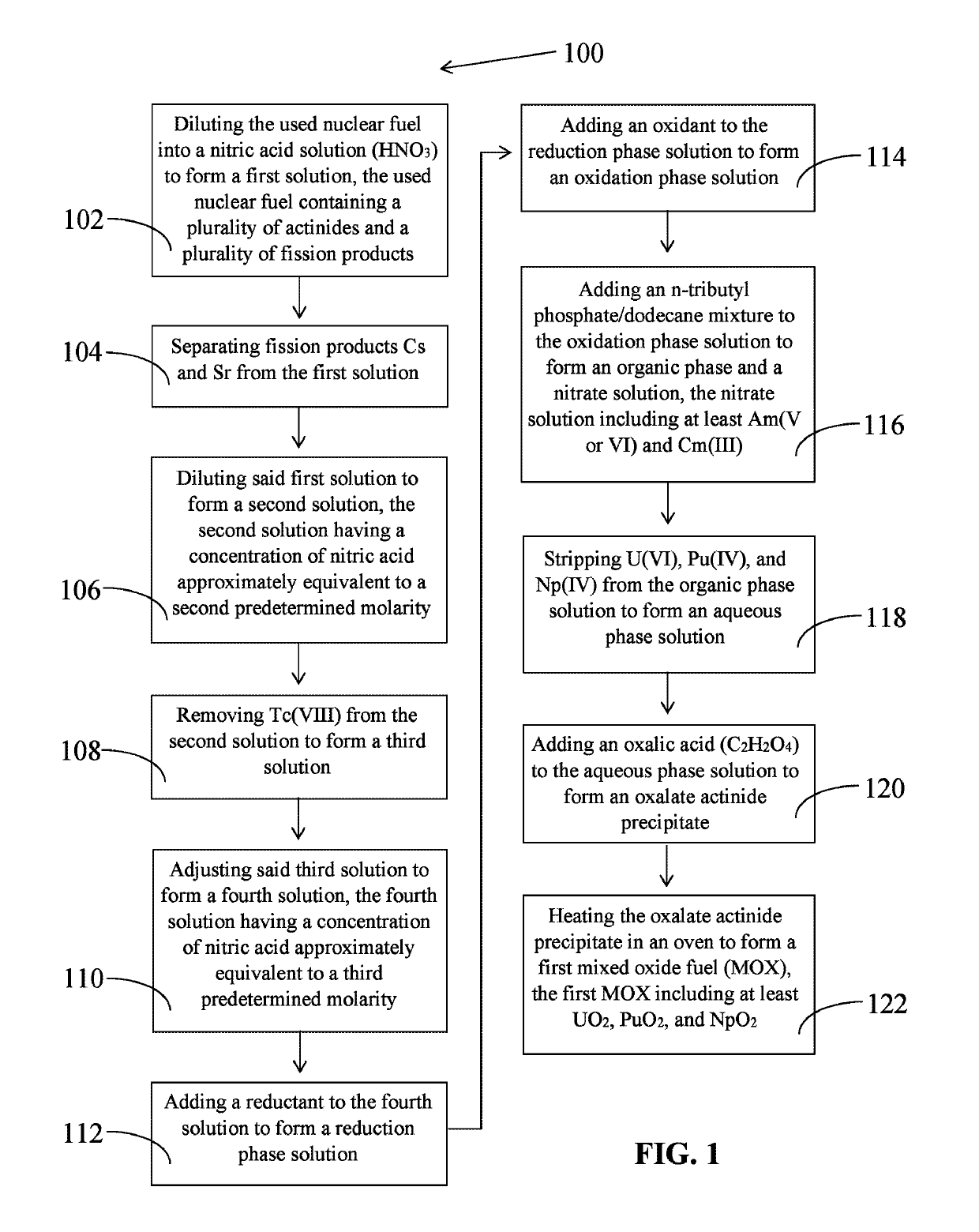
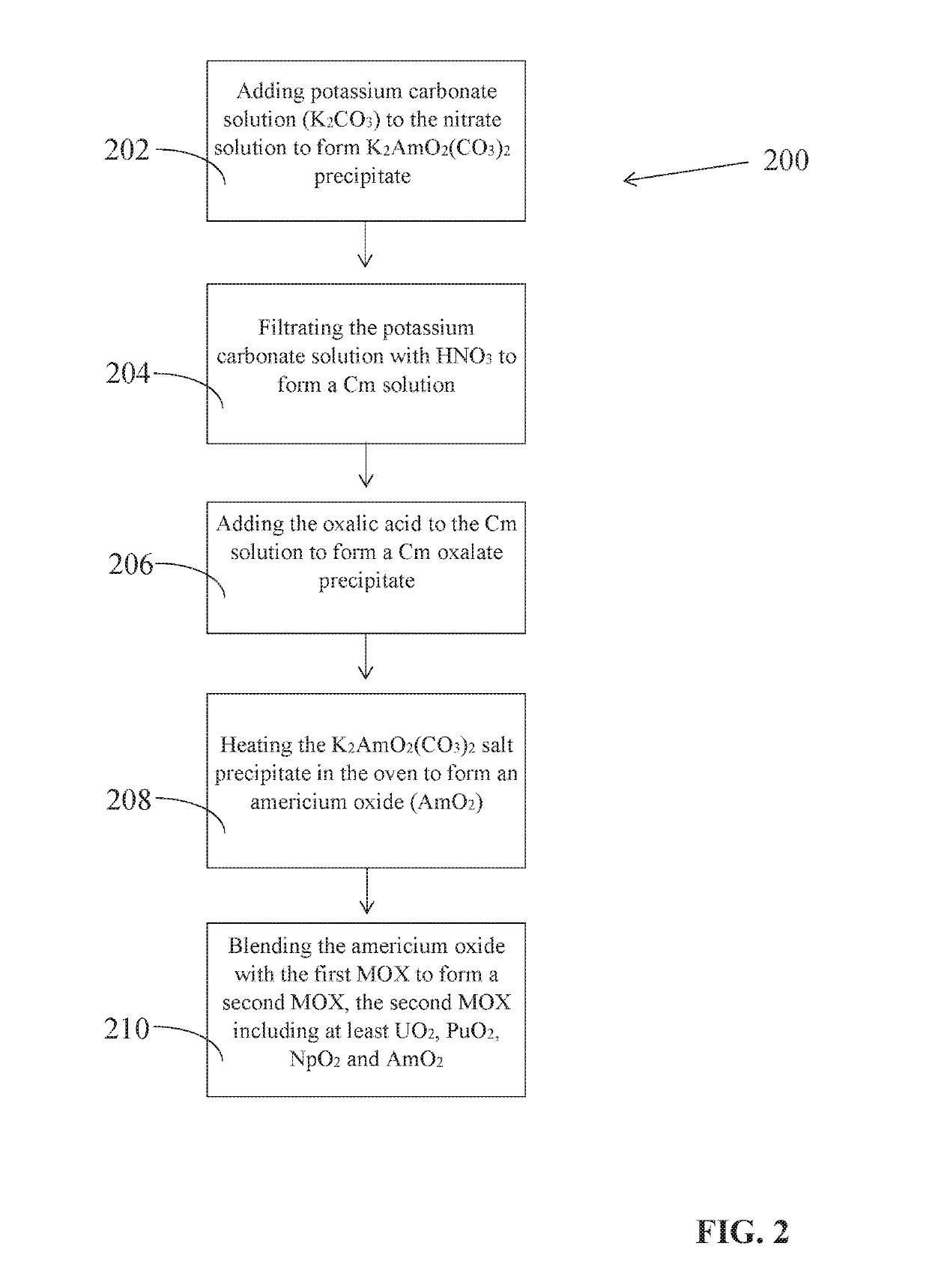
The invention relates to the ROANEX method, which extracts actinides from used nuclear fuel in a single purification cycle. The used nuclear fuel contains actinides, U, Am, Pu, Np. and Cm, and fission products, Cs, Sr and Tc. The fission products are separated first from the used nuclear fuel. The actinides are reduced to their lowest oxidation states and then oxidized to their highest oxidations states. Uranium, Pu and Np move to an organic phase solution and Am and Cm move to a nitrate solution. Uranium, Pu, and Np are stripped from the organic phase solution, and then treated with an oxalic acid to form a precipitate. Americium and Cm are treated with a potassium carbonate solution and Am precipitates. Actinides Am, U, Pu, and Np precipitates are heated in an oven and then blended together to form a mixed oxide fuel of UO2, PuO2, NpO2 and AmO2.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Methods of fabricating metallic fuel from surplus plutonium
A method of fabricating metallic fuel from surplus plutonium may include combining plutonium oxide powder and uranium oxide powder to obtain a mixed powder with reduced proliferation potential. The mixed powder may be electroreduced in a bath of molten salt so as to convert the mixed powder to a first alloy. The first alloy may be pressed to remove a majority of the molten salt adhered to the first alloy to form a pressed alloy-salt mixture. The first alloy may be isolated from the salt by melting the pressed alloy-salt mixture. The first alloy may be further processed to fabricate a fuel rod. Accordingly, the metallic fuel produced may be used in a fast reactor system, such as a Power Reactor Innovative Small Module (PRISM).
Owner:GE HITACHI NUCLEAR ENERGY AMERICAS
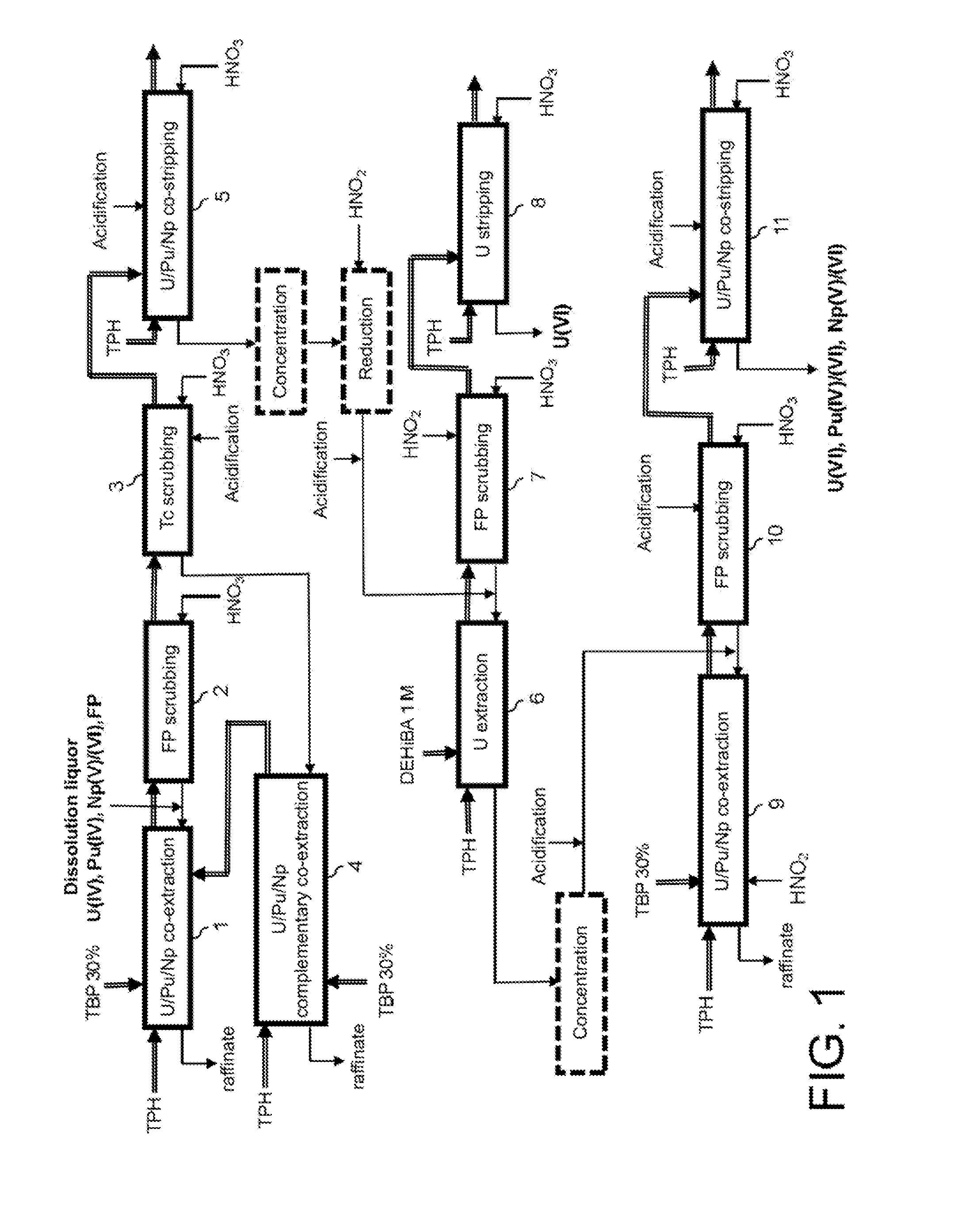
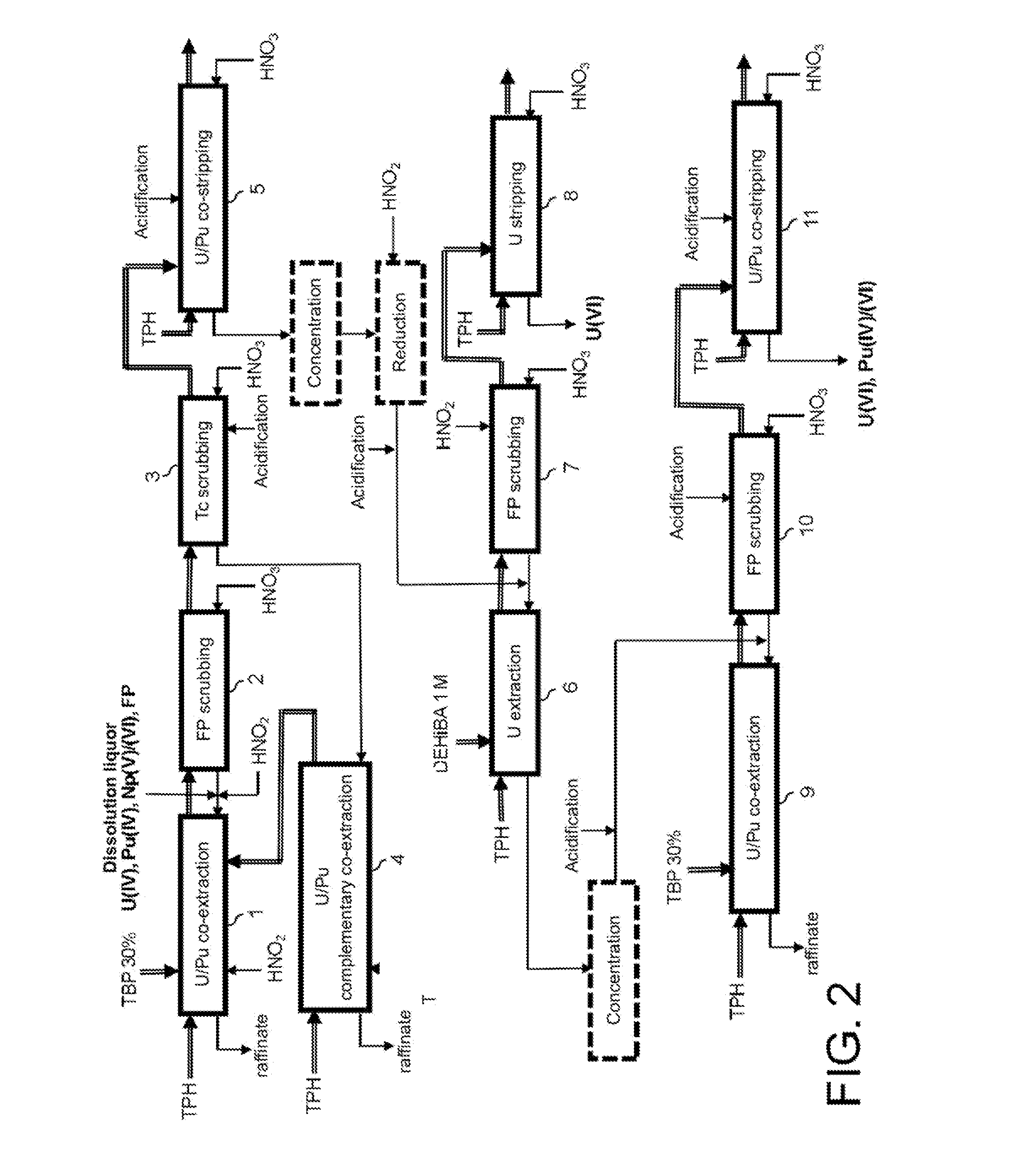
Process for reprocessing spent nuclear fuel not requiring a plutonium-reducing stripping operation
ActiveUS8795610B2Simplify the management processLower the volumeMetal recyclingNuclear fuel reprocessingMixed oxideUranium oxide
The invention relates to a process for reprocessing spent nuclear fuel which, among other advantages, does not require a plutonium-reducing stripping operation.This process finds particular application in the processing of uranium oxide fuels and uranium and plutonium mixed oxide fuels.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
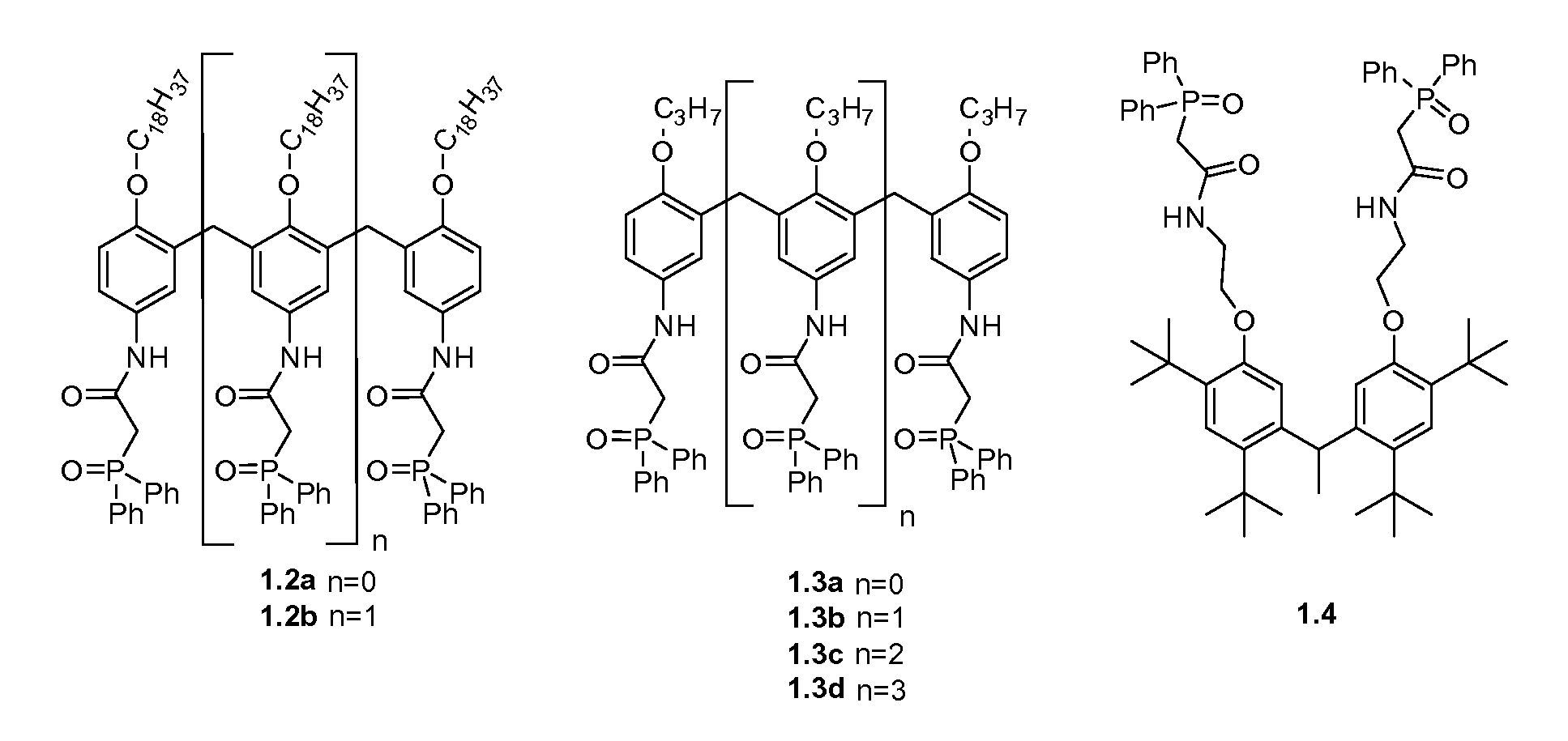
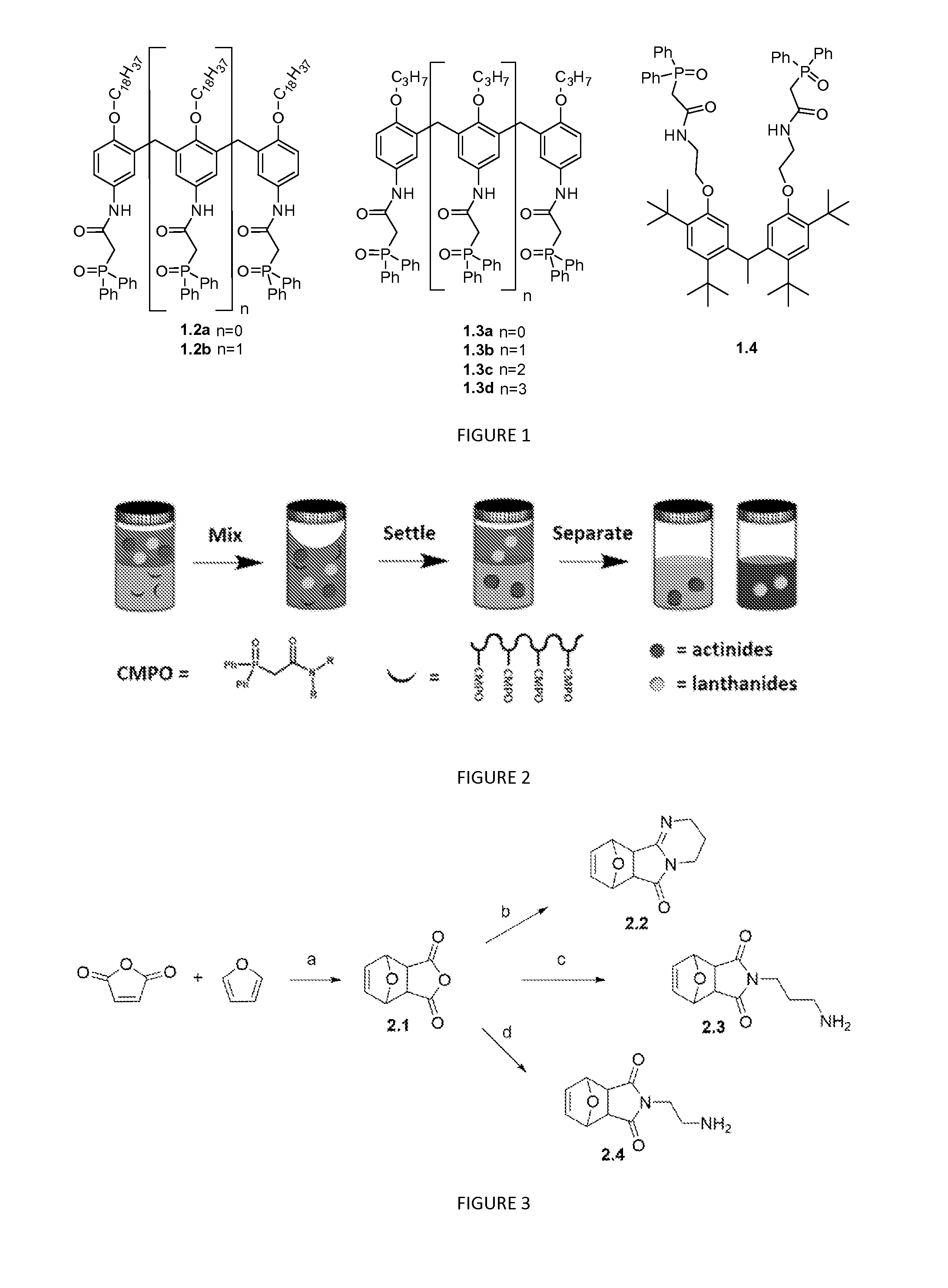
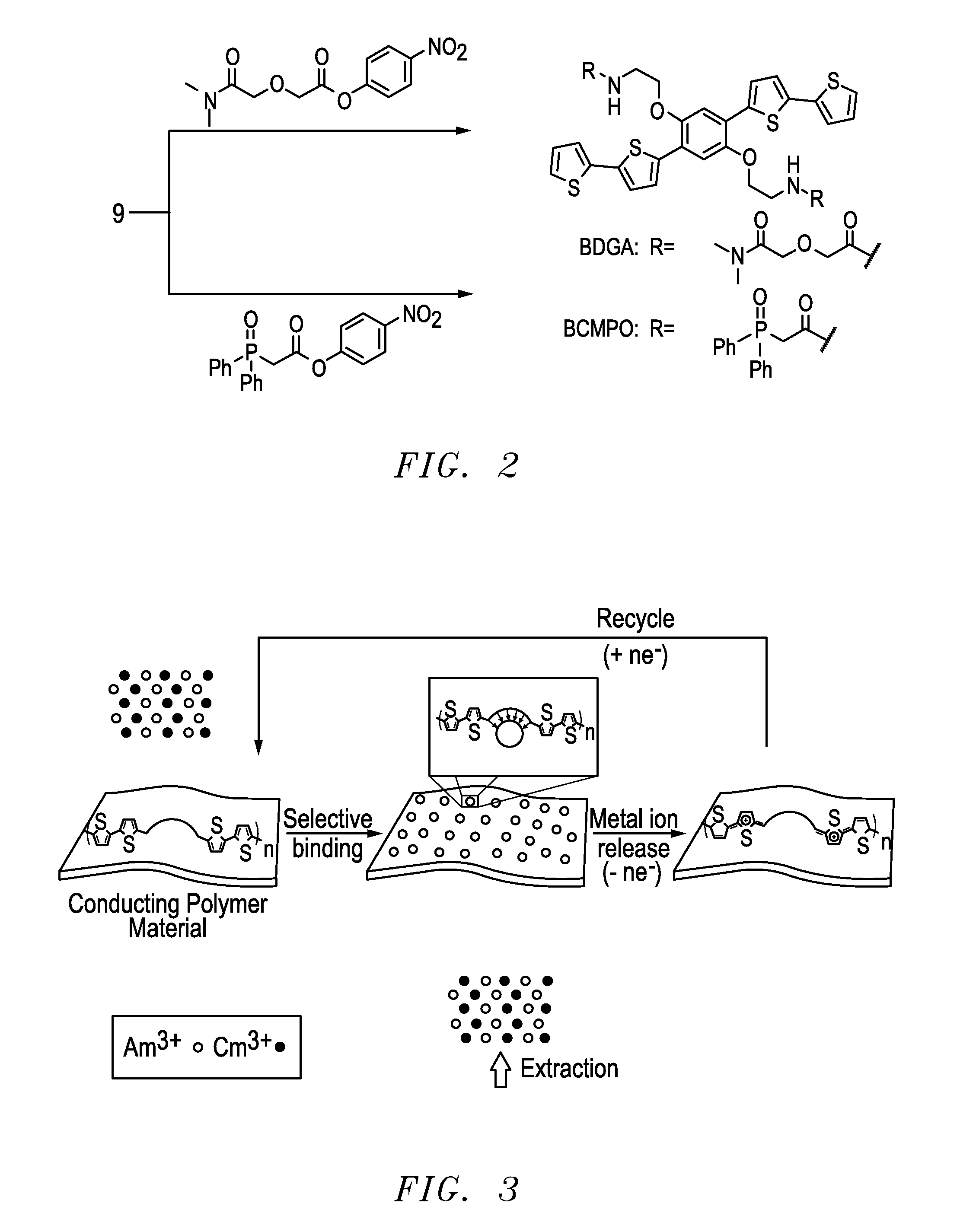
Polymeric chelators for metal ion extraction and separation
InactiveUS20160225472A1Promote absorptionHigh selectivityNuclear fuel reprocessingNuclear energy generationPolymer scienceSide chain
The present invention provides a polymeric extractant with pendant chelator groups for selective sequestration of actinides and / or lanthanides comprising: a first block polymer comprising one or more CMPO monomers each having a first backbone bound to a pendant carbomylmethylphosphine oxide group; a second block polymer comprising one or more second monomers each having a second backbone bound to a second pendant group, wherein the first backbone is polymerized to the second backbone to form a polymer backbone with pendent blocks of the second pendant group and pendent blocks of pendent carbomylmethylphosphine oxide group that sequester the actinides and / or lanthanides; and optionally a third block polymer comprising one or more third monomers each having a third backbone bound to a third pendant group, wherein the one or more third monomers is polymerized to the first backbone and the second backbone.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for reprocessing spent nuclear fuel and centrifugal extractor therefor
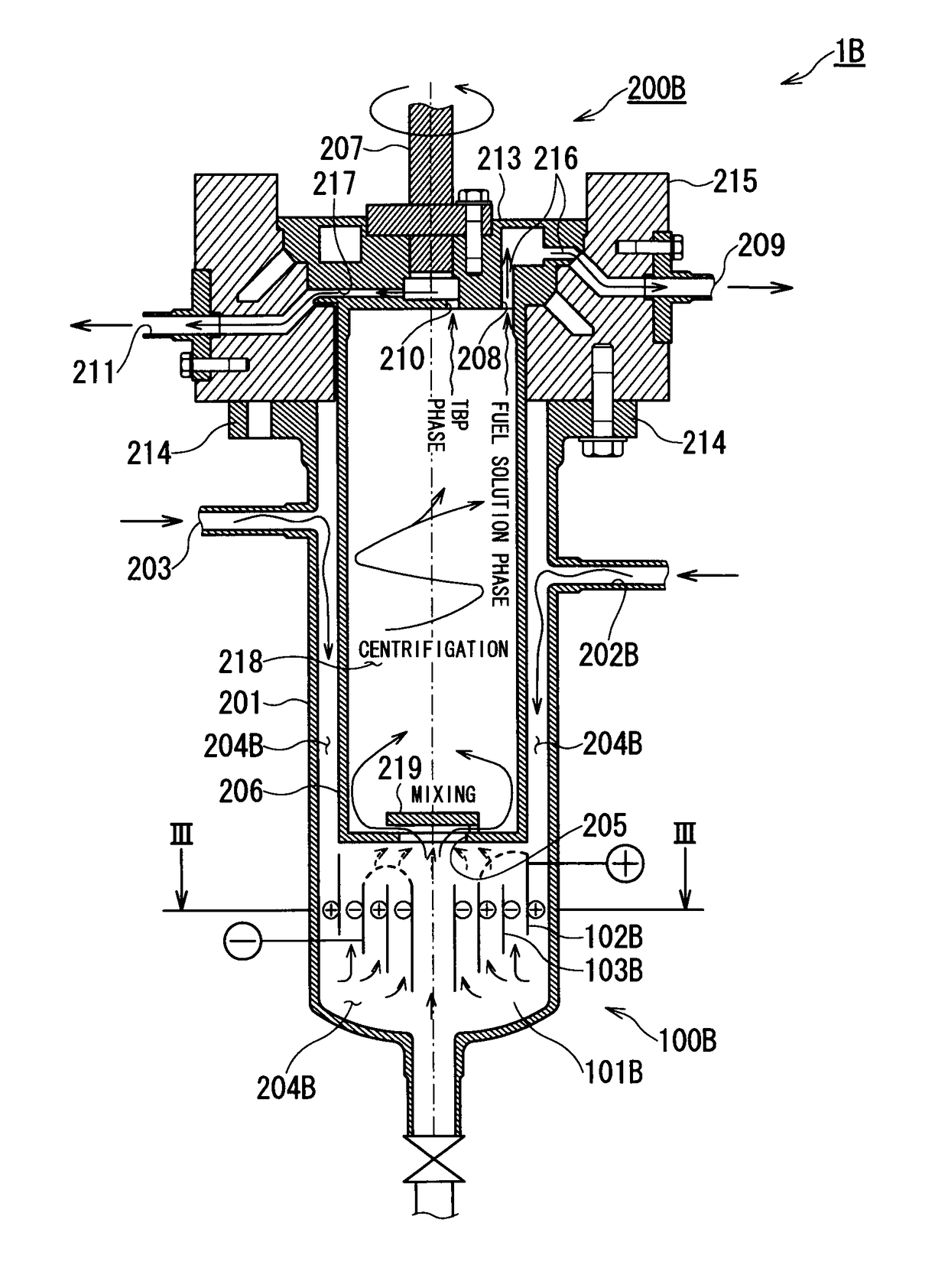
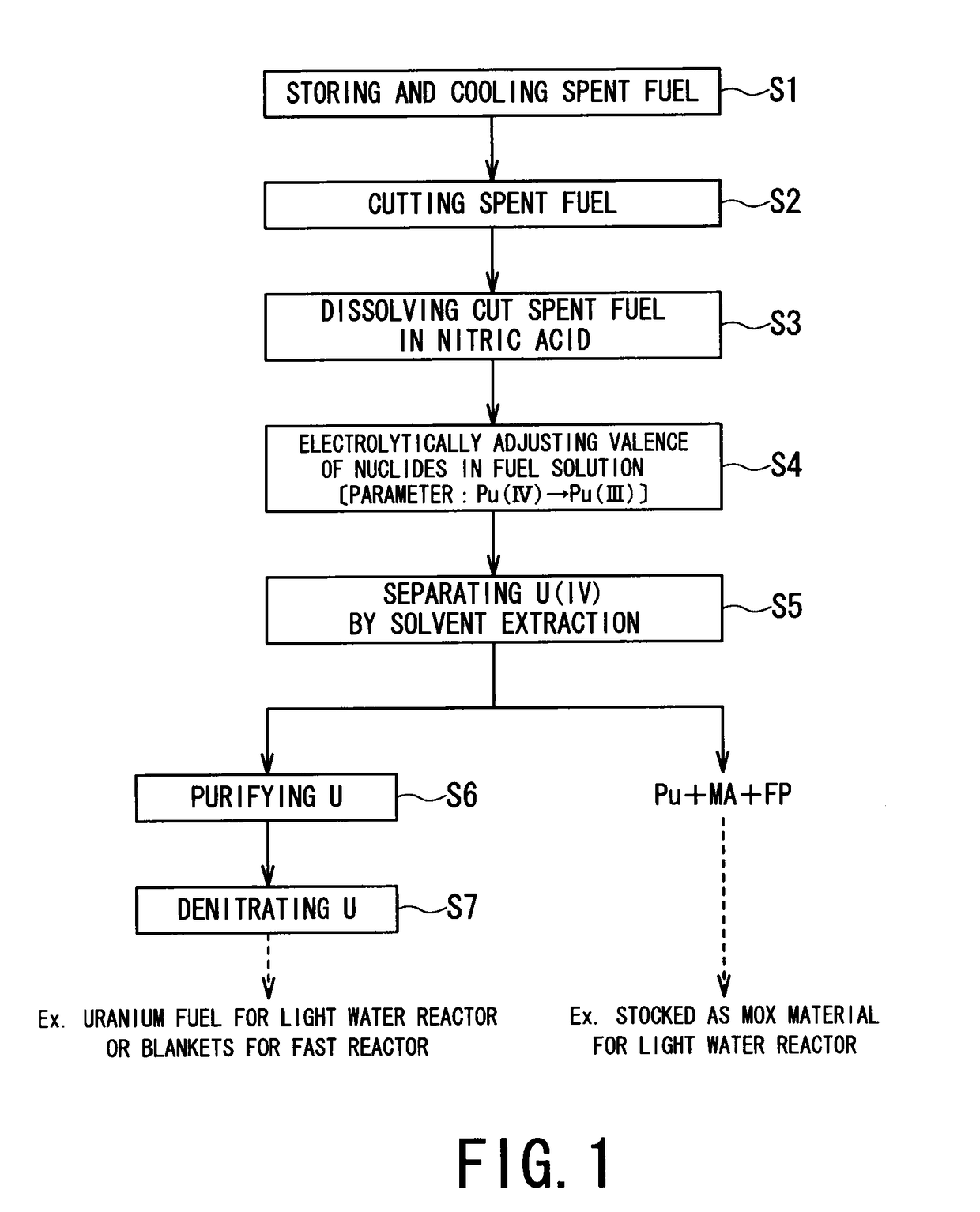
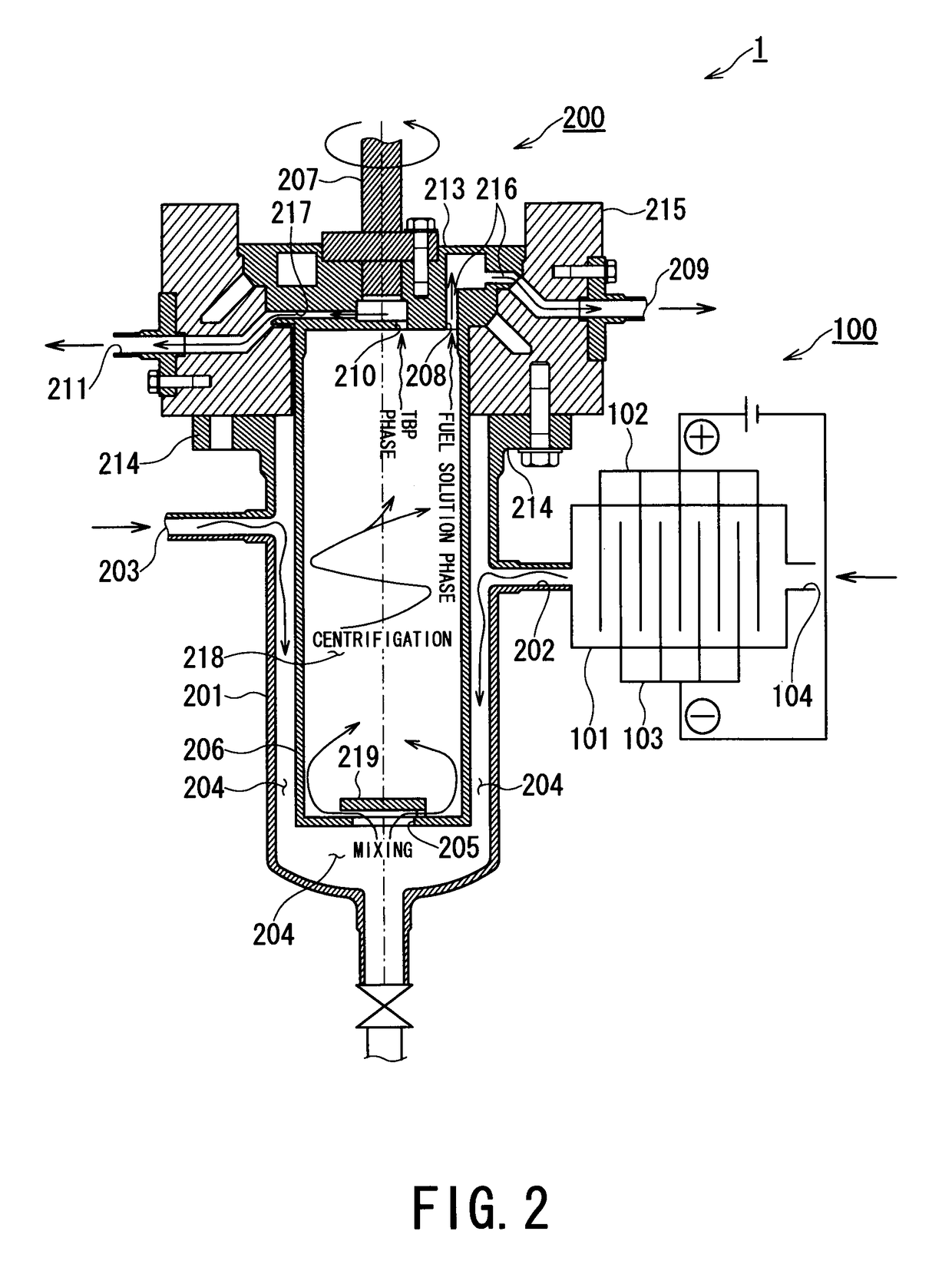
ActiveUS9666315B2Isolation difficultRecovery difficultWater/sewage treatment by centrifugal separationNuclear fuel reprocessingElectrolysisEngineering
A spent nuclear fuel is reprocessed by dissolving a spent nuclear fuel in an aqueous nitric acid solution and separating and recovering nuclides contained in the resulting fuel solution by solvent extraction. A spent nuclear fuel reprocessing method includes: an electrolytic valence adjustment step in which nuclides contained in the fuel solution is electrolytically reduced without removing fission products or minor actinides until valence of plutonium is at a level at which solvent extraction efficiency is low by using the valence of plutonium contained in the fuel solution as a parameter; and a nuclide separation step in which, by using an extraction solvent which extracts uranium contained in the fuel solution, uranium is distributed from the fuel solution subjected to the electrolytic valence adjustment step to the extraction solvent.
Owner:KK TOSHIBA
Method for purifying the uranium from a natural uranium concentrate
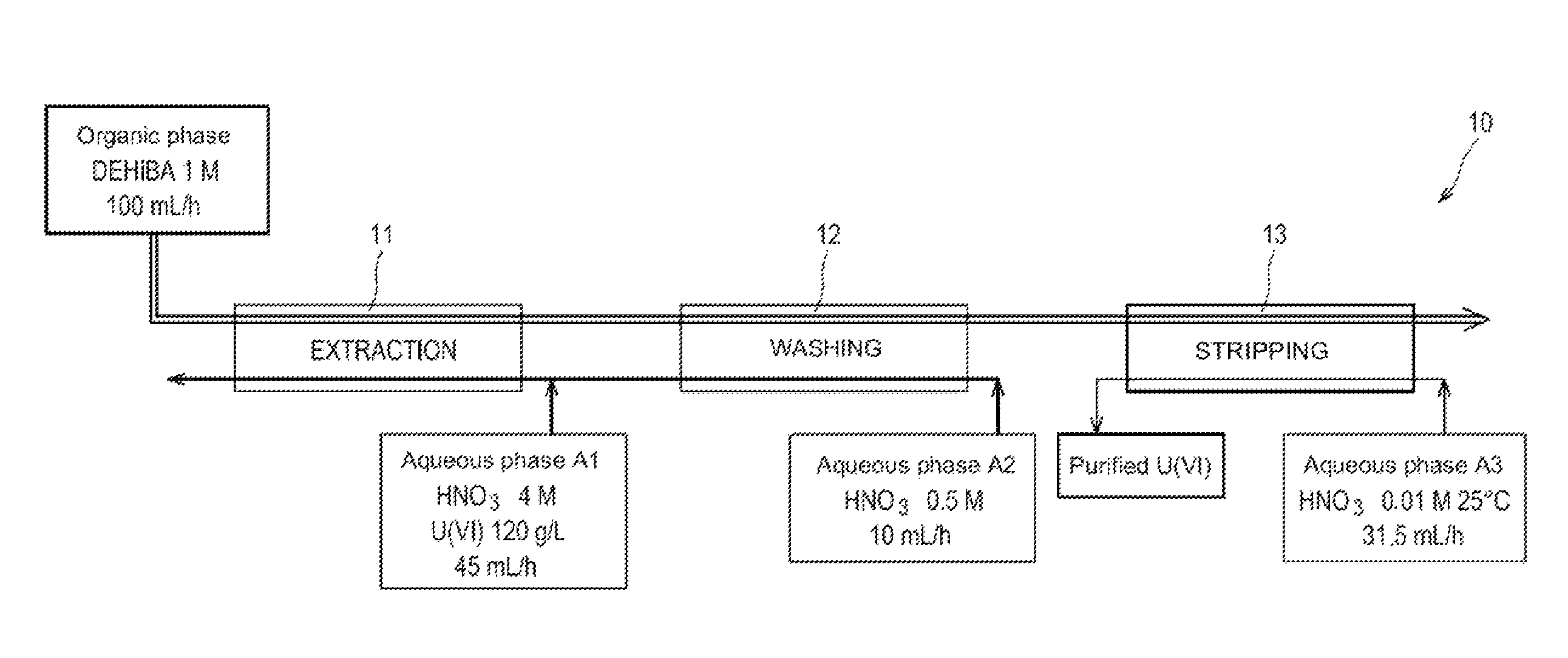
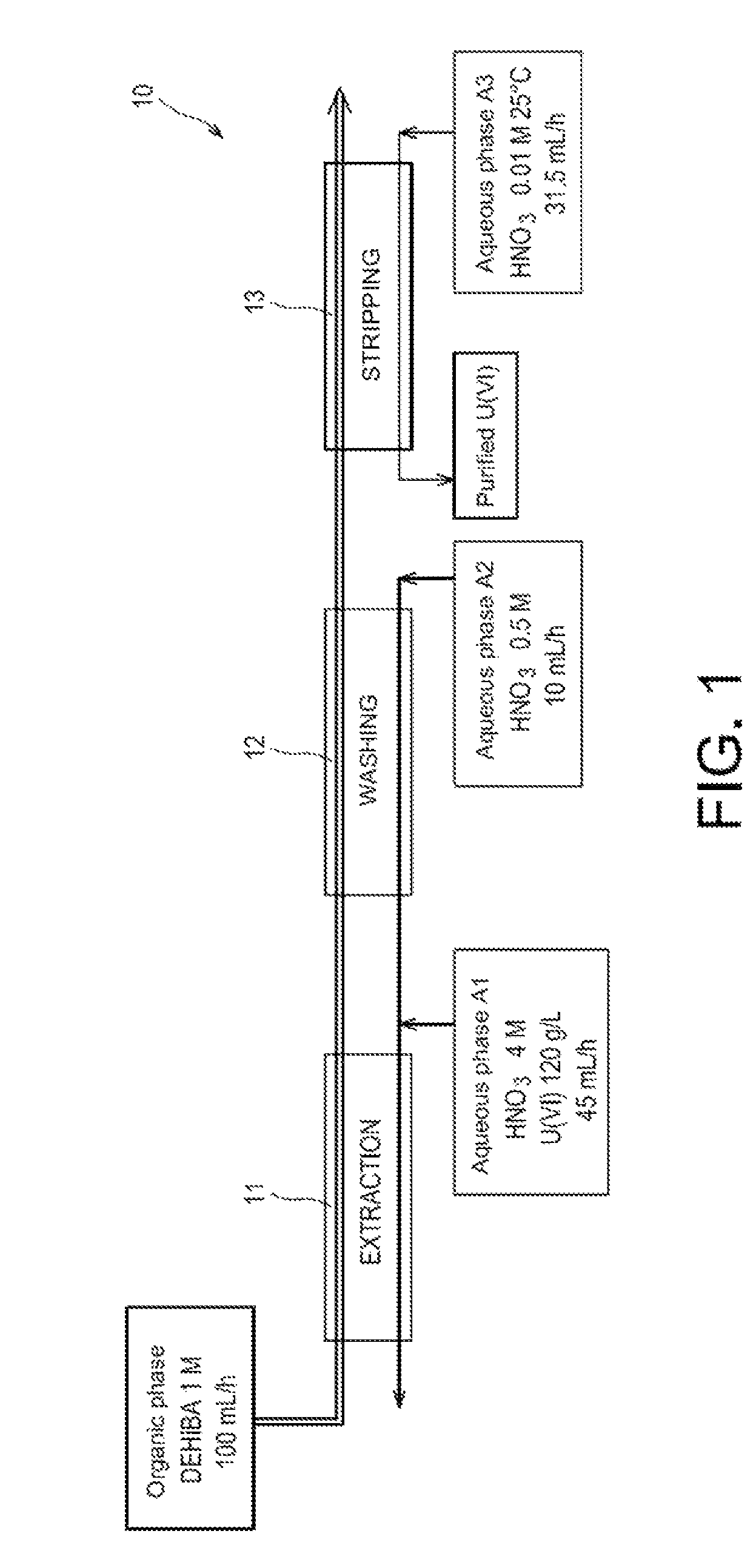
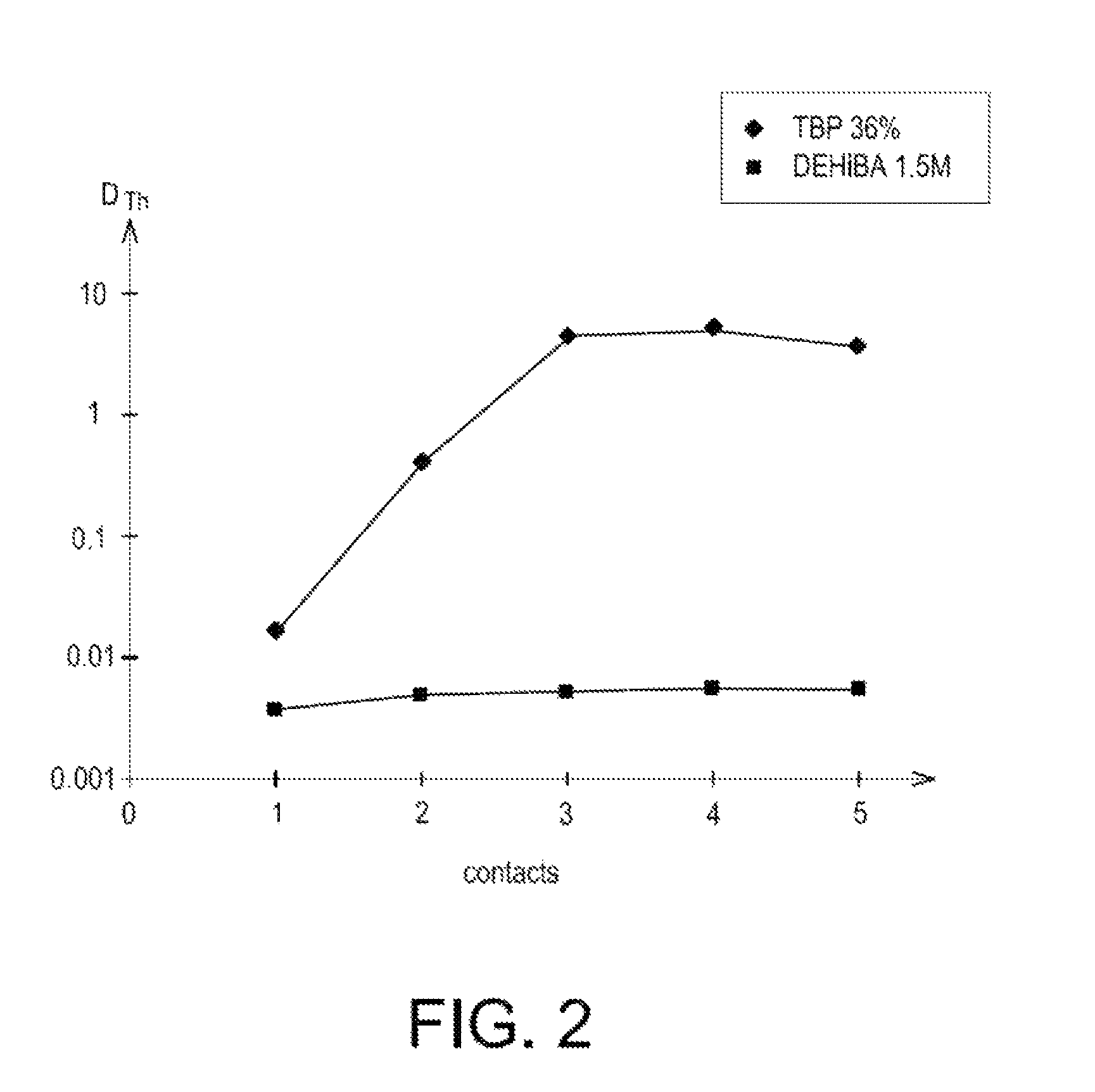
ActiveUS8795611B2Simplification of exploitationEasy to processNuclear fuel reprocessingSolvent extractionNatural uraniumDiluent
A method with which uranium from a natural uranium concentrate may be purified, includinga) extracting the uranium present as uranyl nitrate in an aqueous phase A1 resulting from the dissolution of the natural uranium concentrate in nitric acid, by means of an organic phase which contains an extractant in an organic diluent;b) washing the organic phase obtained at the end of step a), with an aqueous phase A2; andc) stripping the uranyl nitrate of the organic phase obtained at the end of step b), by circulating this organic phase in an apparatus, as a counter current against an aqueous phase A3.The extractant is an N,N-dialkylamide and the ratio between the flow rate at which the organic phase obtained at the end of step b) and the aqueous phase A3 circulate in the apparatus where step c) occurs, is greater than 1.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
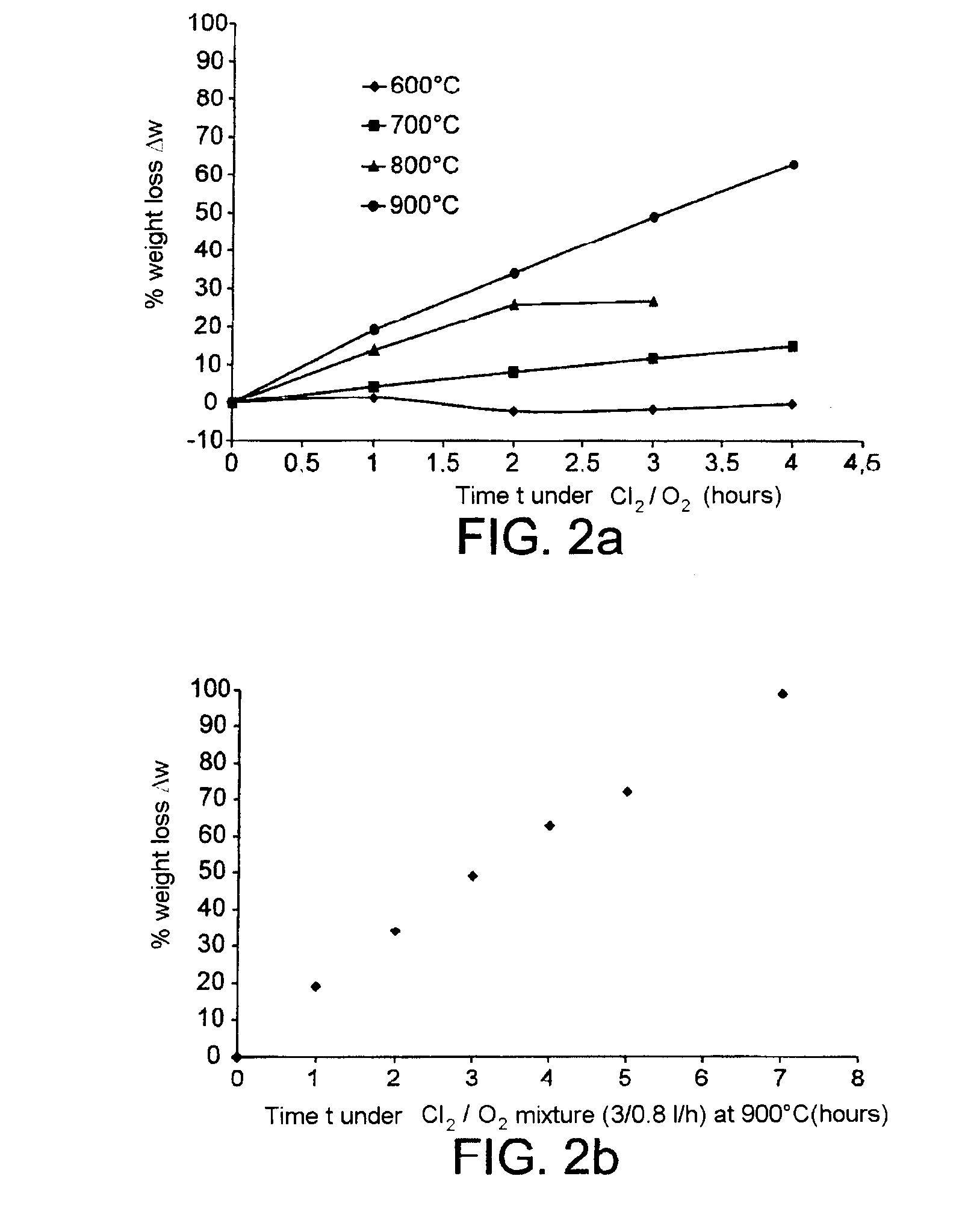
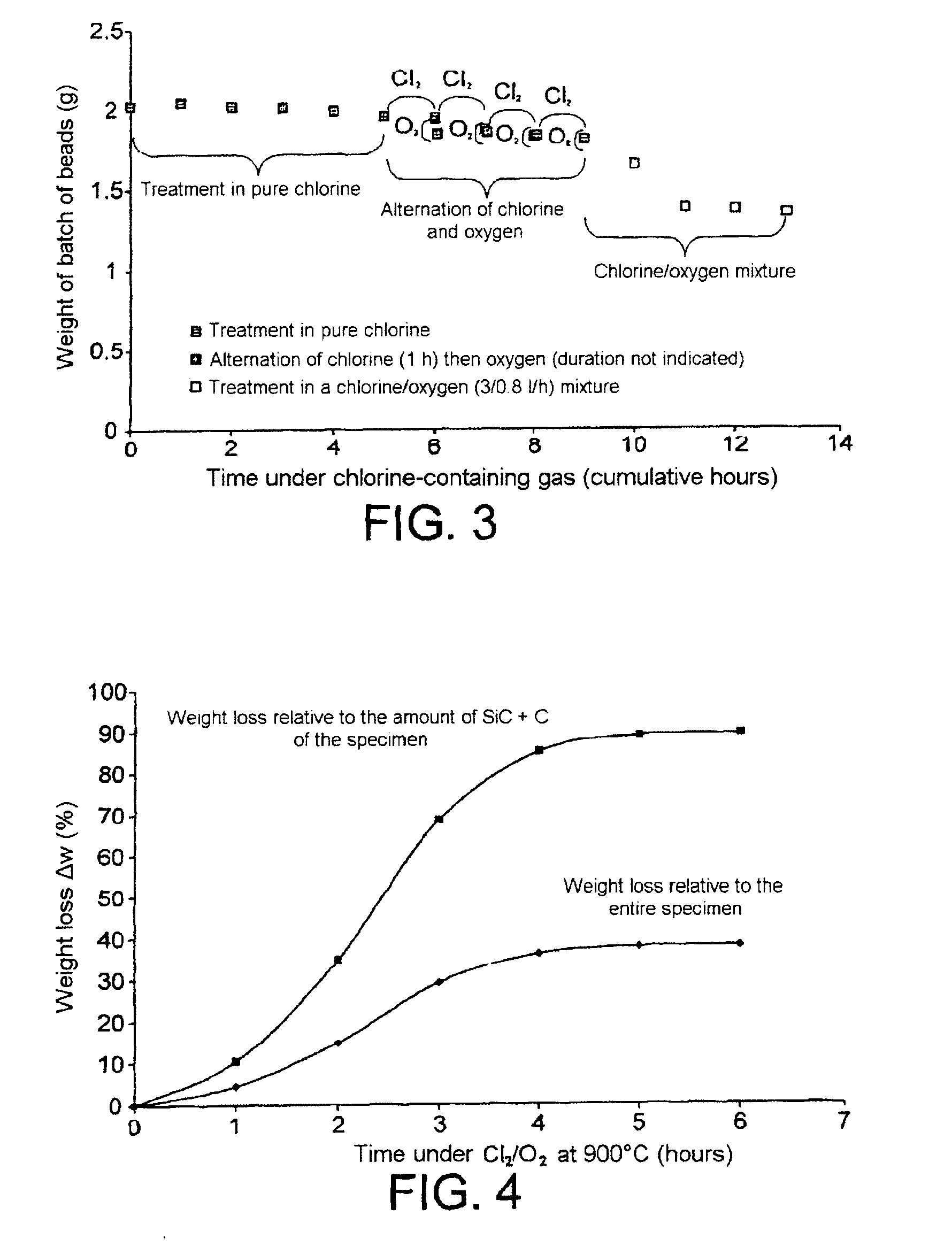
Method for processing nuclear fuels containing silicon carbide and for decladding nuclear fuel particles
InactiveUS20100314592A1Short cooldownNuclear fuel reprocessingNuclear energy generationOxygen mixtureMaterials science
The present invention relates to a method for processing a nuclear fuel comprising a fissile material, SiC and possibly carbon, said method comprising the contacting of said fuel with a chlorine / oxygen mixture at a temperature below 950° C., and more particularly at a temperature between 400 and 900° C., so as to remove the SiC, and the carbon if this is present, from said fuel. The method of the invention makes it possible for example to declad TRISO or BISO nuclear fuel particles, i.e. particles enabling the nuclear fuel to be confined in a sheath or cladding, or to remove an SiC matrix from a fuel having a heterogeneous SiC matrix. The present invention therefore has many applications, especially in the reprocessing of irradiated nuclear fuels.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Functional conducting polymers for redox mediated separations of f-elements
InactiveUS20160225471A1Reduce technical burdenImprove efficiencyNuclear fuel reprocessingNuclear energy generationTerthiopheneRedox
The present invention provides a redox mediated polymer composition for the selective sequestration and separation of multivalent metal ions comprising: 2 or more monomers polymerized to form a polymeric thiophene backbone, wherein each of the 2 or more monomers comprise 2 thiophene portion groups for polymerization and 2 pendant carbomylmethyl-phosphine oxide or diglycolamide groups that sequester selectively the multrivalent metal ions.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
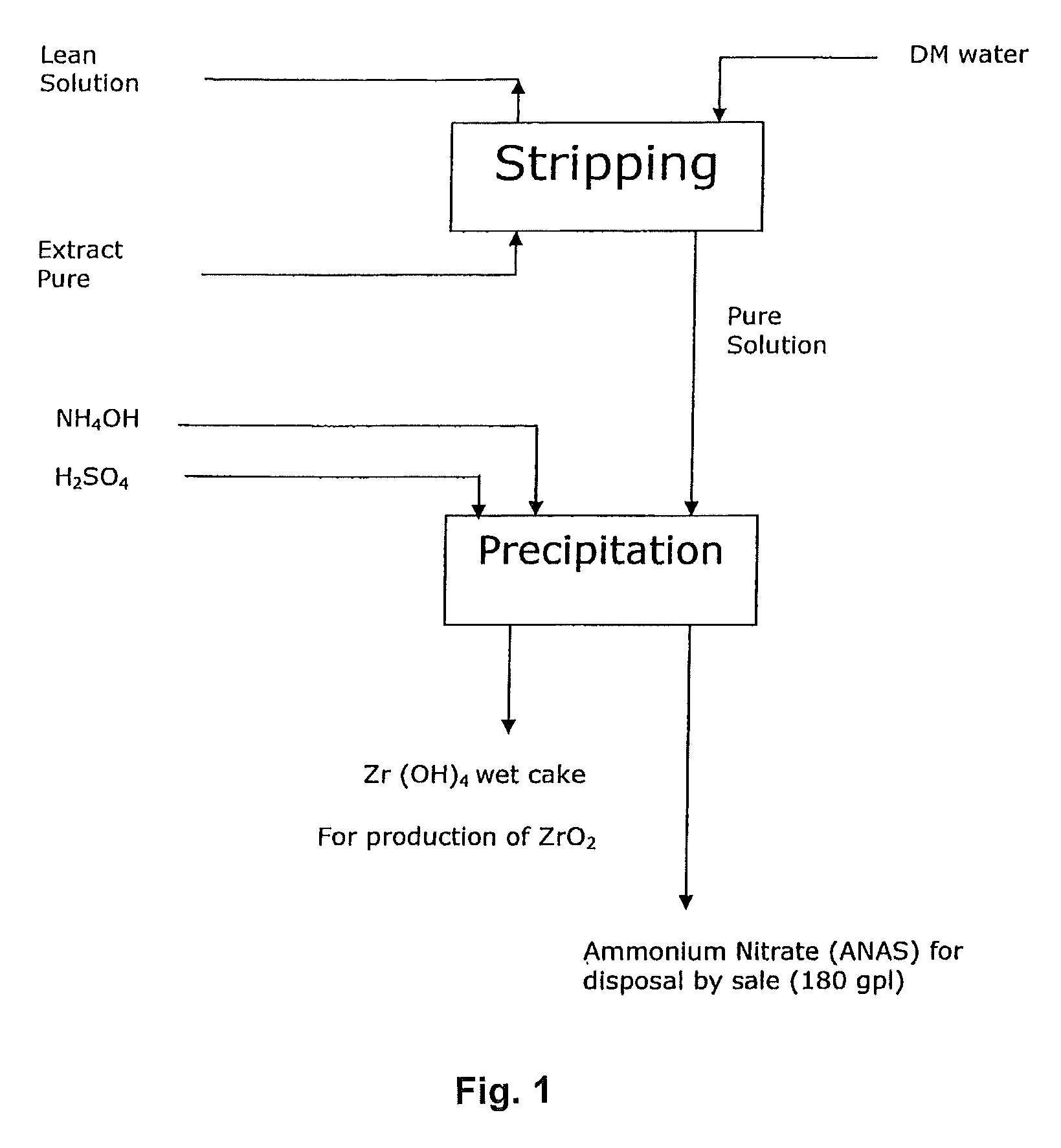
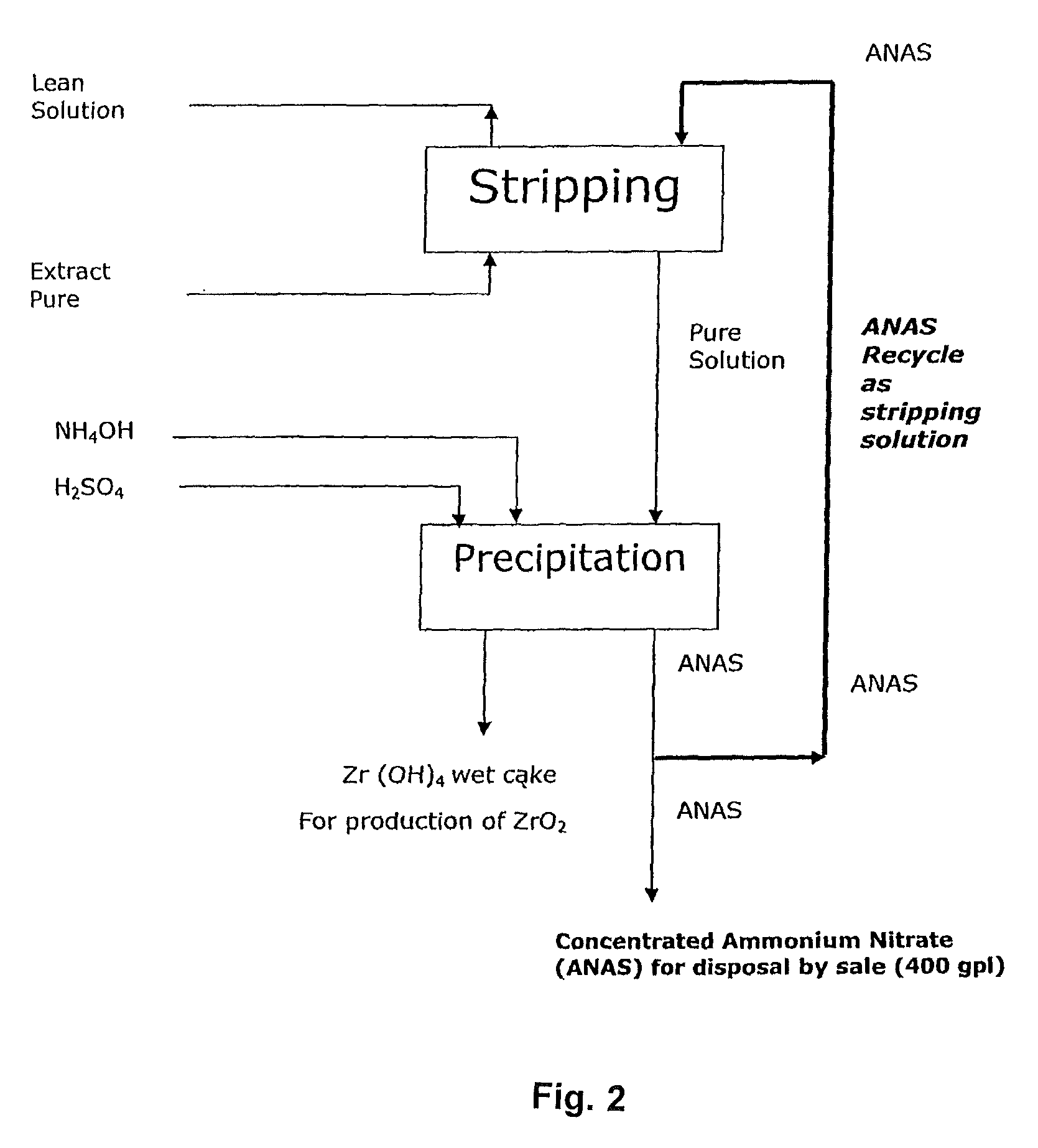
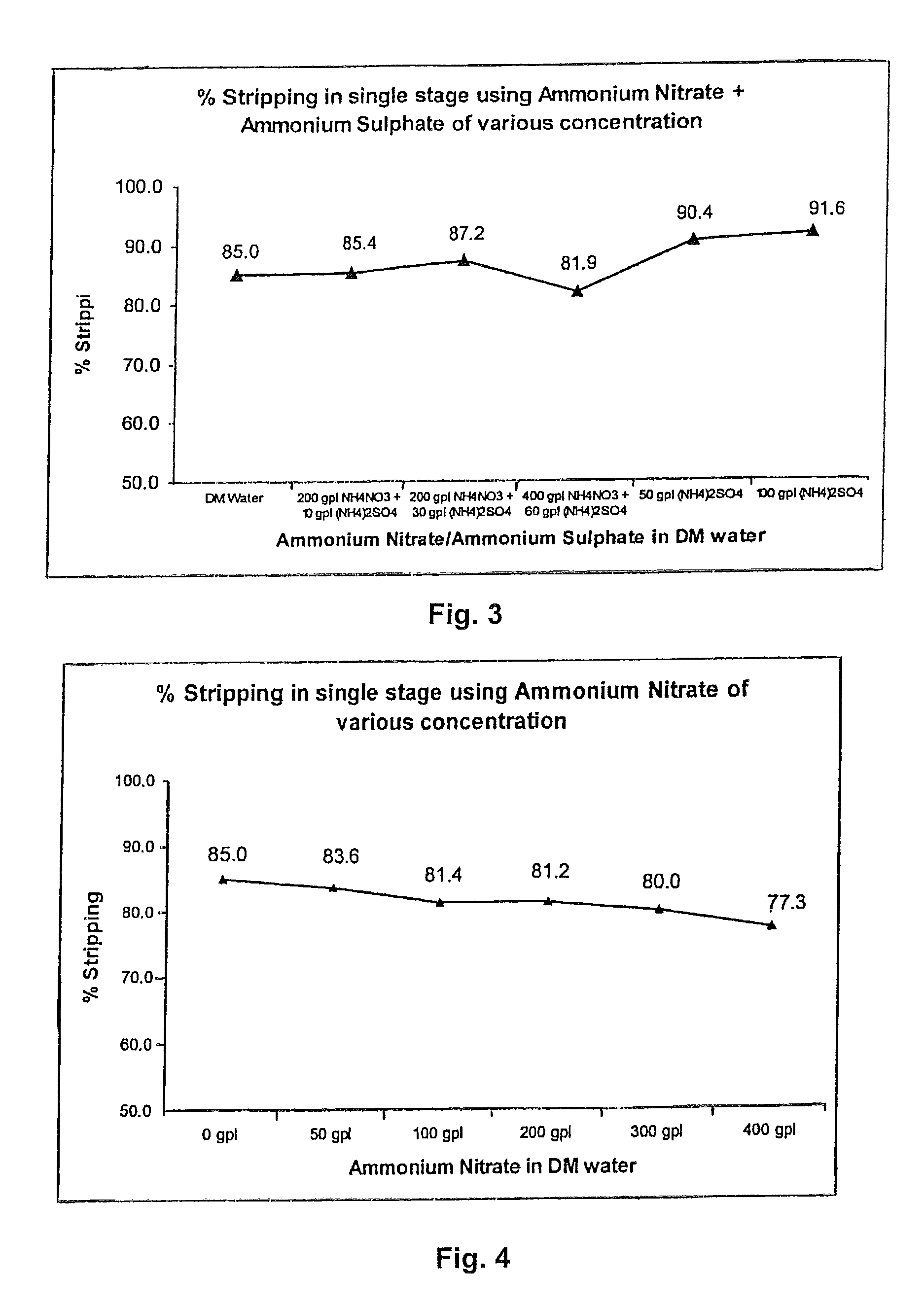
Tributyl phosphate-nitrate solvent extraction process for producing high purity nuclear grade rare earth metal oxides
InactiveUS9174855B2Reduce generationImprove concentrationNuclear fuel reprocessingUranium compounds preparationOrganic solventRare earth
An improved process for the preparation of high purity rare metal compounds such as oxides utilizing TBP (Tri-Butyl Phosphate)-nitrate solvent extraction technique adapted to manufacture nuclear grade rare metal compounds such as zirconium oxide wherein the said process substantially aids in reducing the specific generation of ammonium nitrate effluent volume thereby increasing its concentration when the said effluent comprising ammonium nitrate and ammonium sulphate are utilized for stripping of the said rare metal compound from the organic solvent in the said process of production of high purity rare metal oxide powder.
Owner:SEC DEPT OF ATOMIC ENERGY
Use of a KMgF.sub.3 compound for trapping metals in the form of fluorides and/or oxyfluorides in a gaseous or a liquid phase
InactiveUS8858901B2Retain these metals very effectivelyEfficient retentionMagnesium fluoridesNuclear fuel reprocessingGas phaseMetal impurities
The invention relates to the use of a compound of the formula KmgF3 to trap metals in the form of fluorides and / or of oxyfluorides in a gaseous or liquid phase. It also relates to a compound of the formula KMgF3 which has a surface area at least equal to 30 m2 / g and at most equal to 150 m2 / g and also to its methods of preparation. The invention notably finds application in the nuclear industry, in which it can advantageously be used to purify uranium hexafluoride (UF6) present in a gaseous or liquid stream, with regard to metal impurities which are also present in this stream.
Owner:COMURHEX
Method for separating and recycling uranium and fluorine form solution
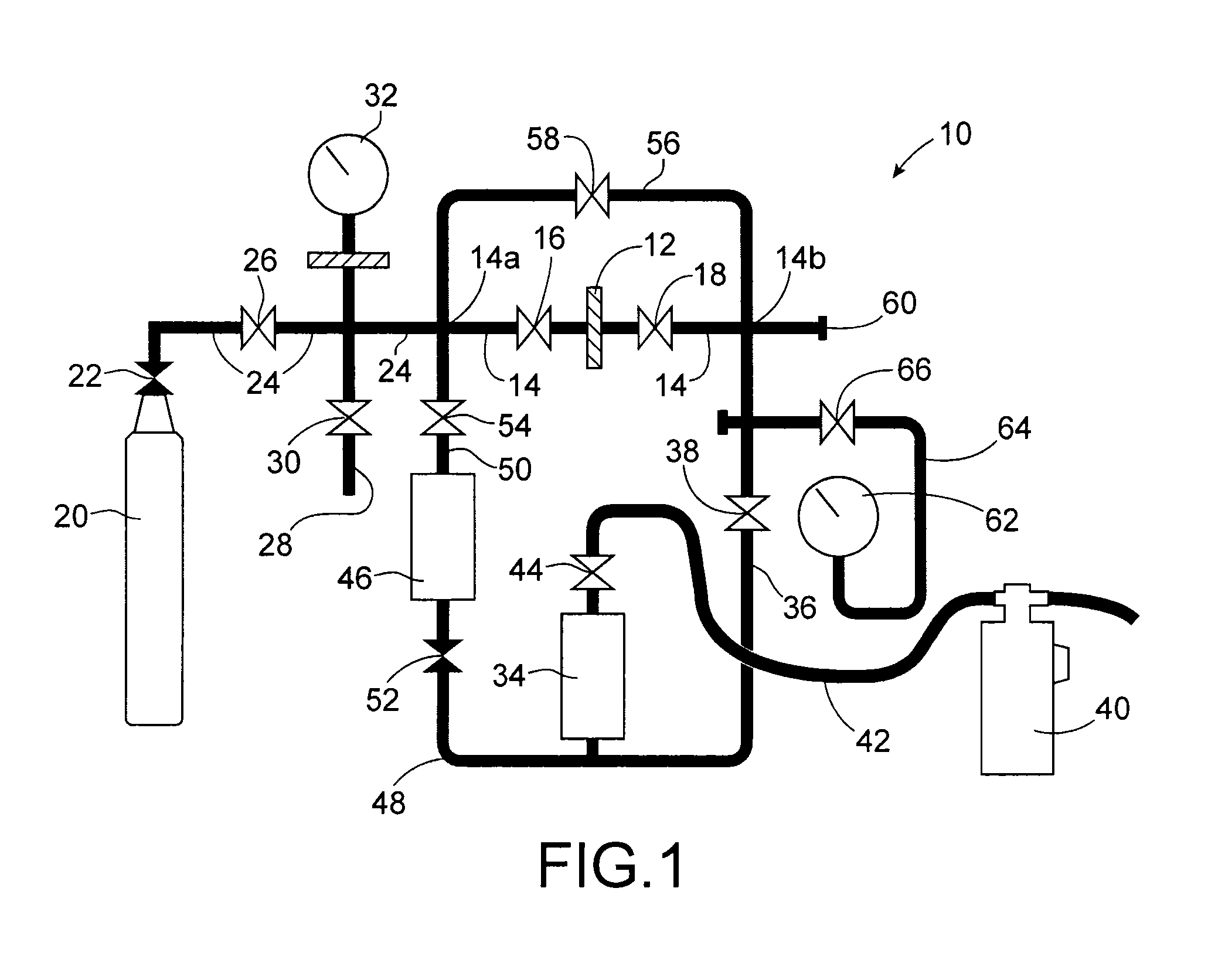
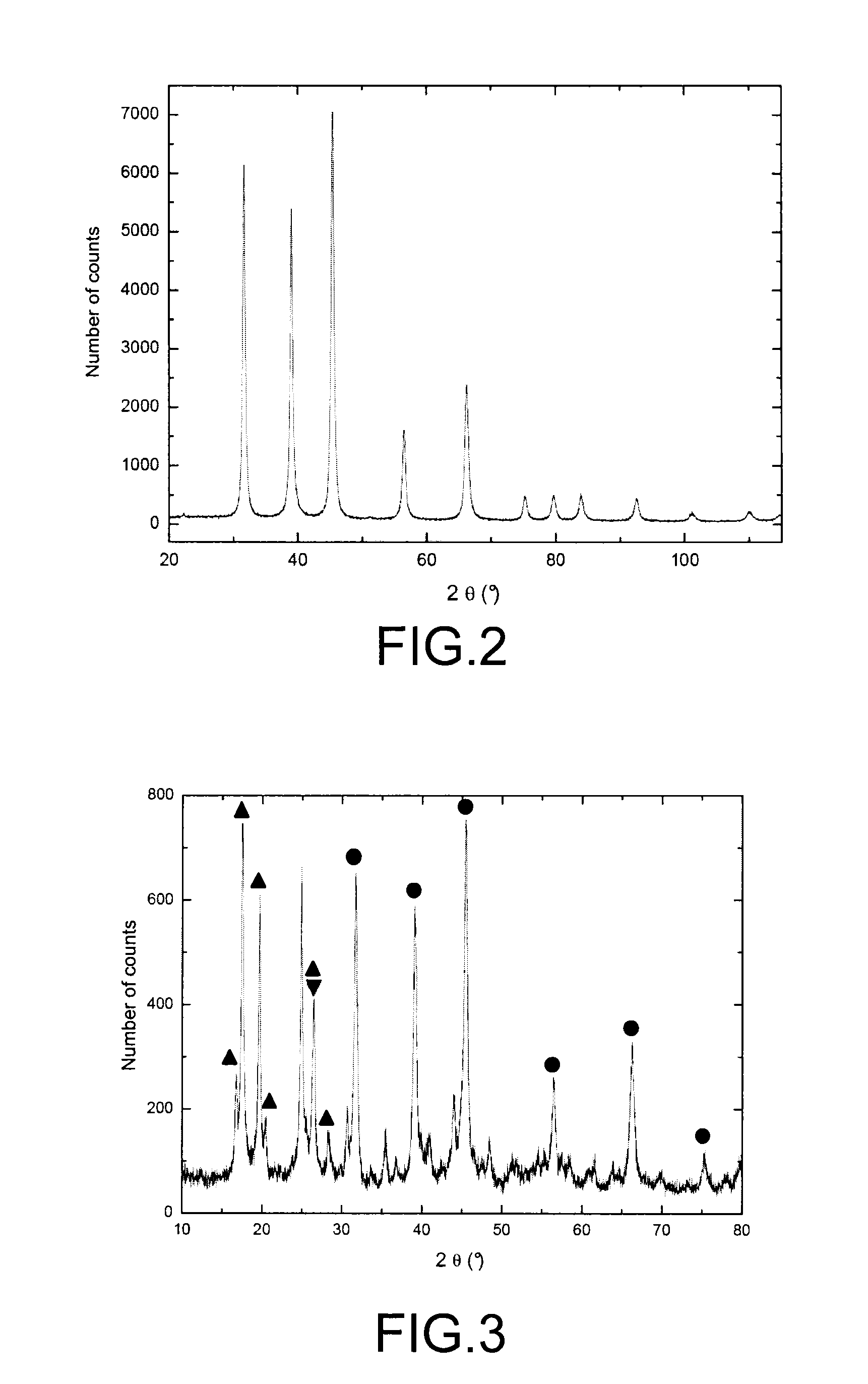
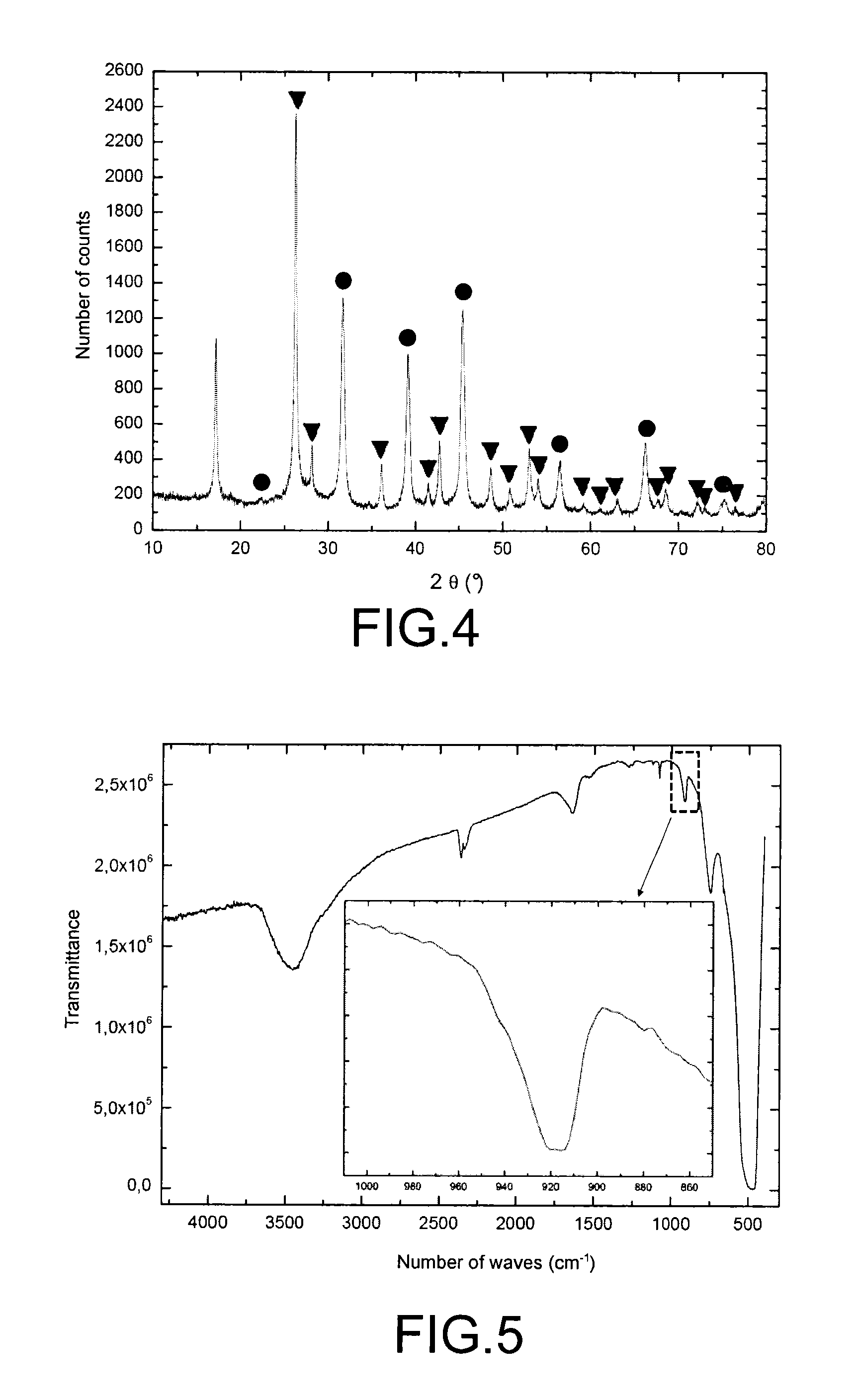
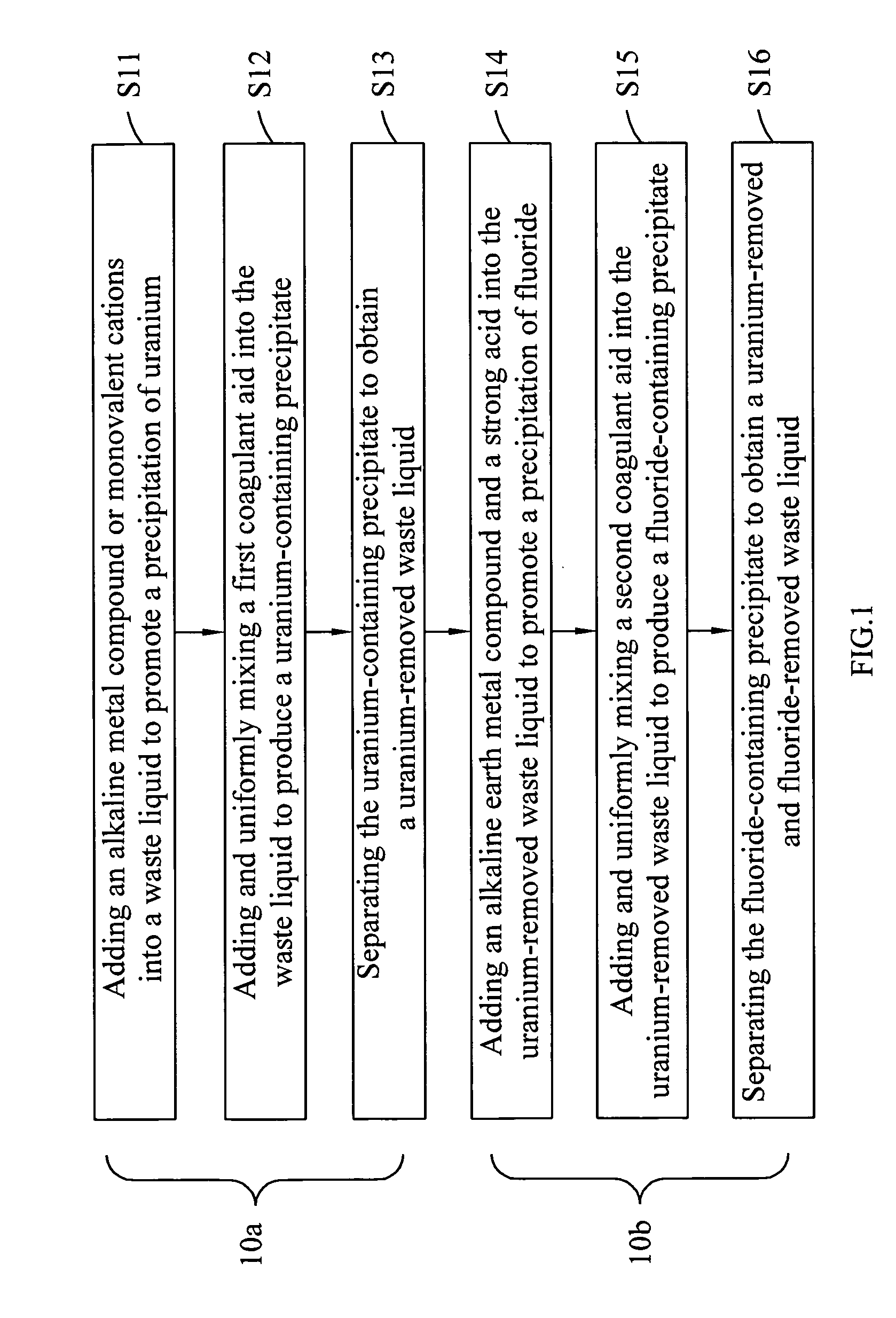
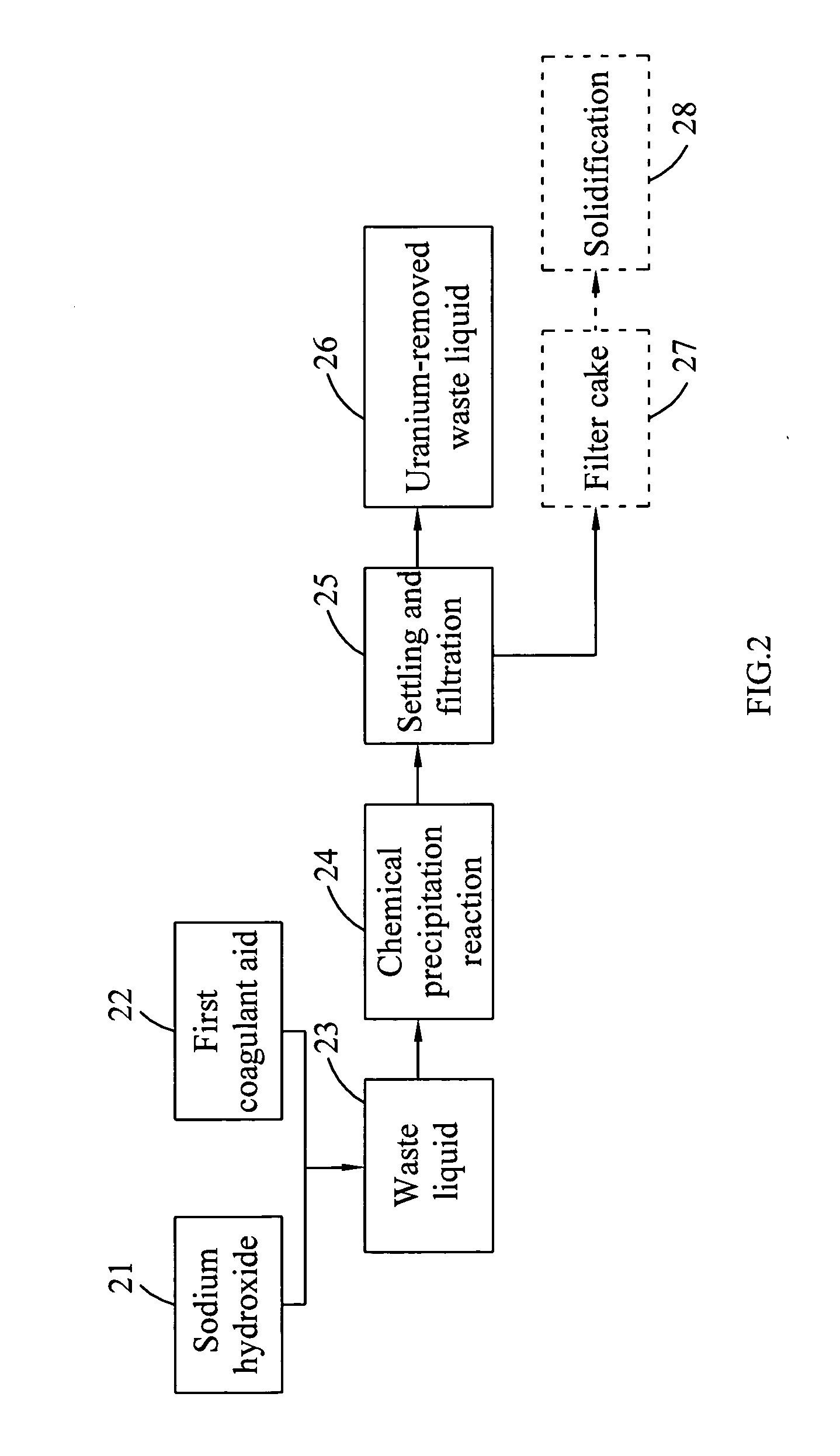
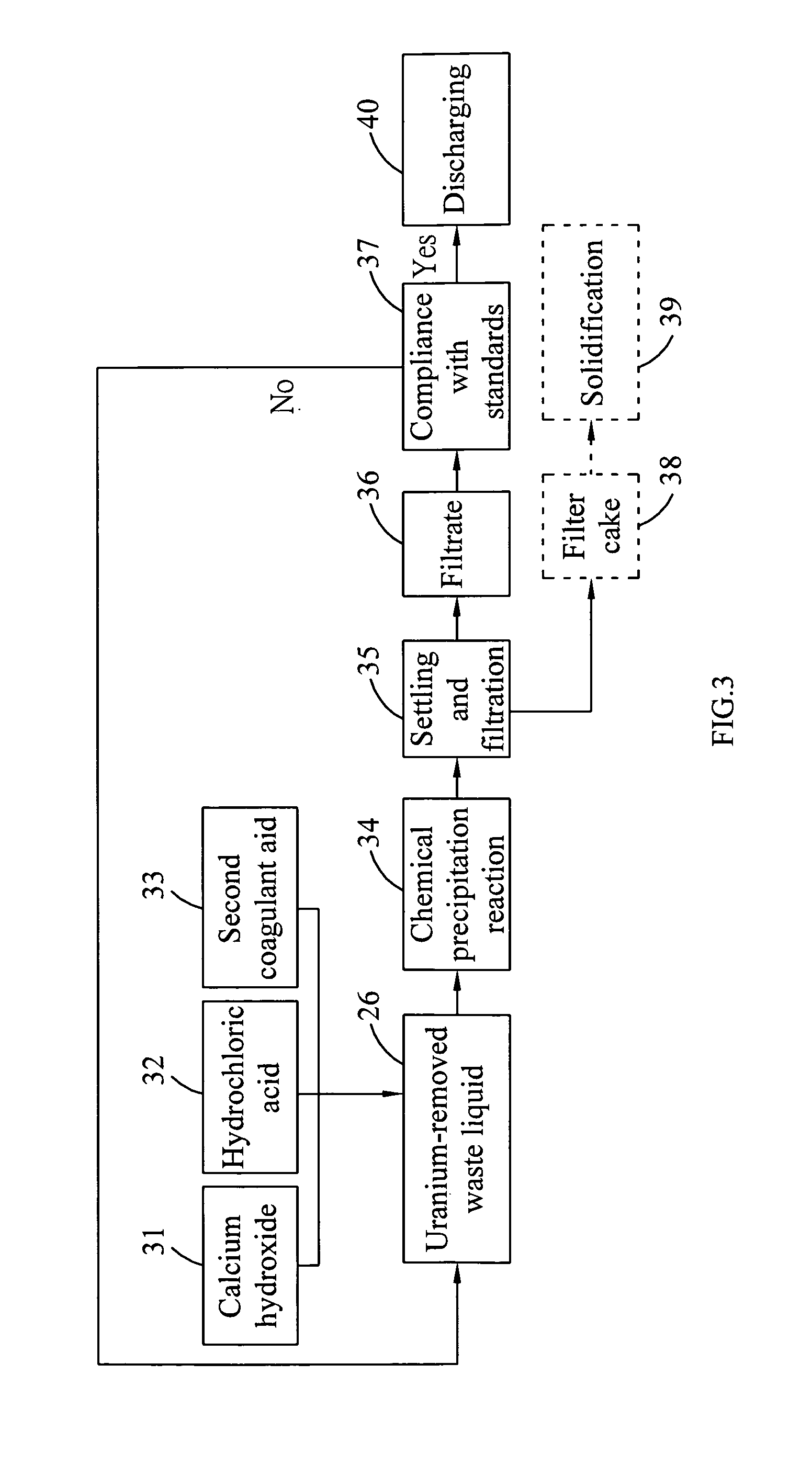
InactiveUS20100316543A1Effective preventionAvoid environmental pollutionNuclear fuel reprocessingTransuranic element compoundsLiquid wasteCyclic process
A separation and recycling method for recycling uranium and fluoride from a waste liquid sequentially and separately is disclosed. The method comprises a uranium-recycling process and a fluoride-recycling process. In the uranium-recycling process, an alkali metal compound or monovalent cation and a coagulant aid are added into the waste liquid to promote the precipitation of uranium. In the fluoride-recycling process, an alkaline earth metal compound, a strong acid and a coagulant aid are added into the uranium-removed waste liquid to precipitate fluoride. By means of the method of the present invention, the uranium and fluoride contents of the uranium-removed and fluoride-removed waste liquid are compliant with the effluent standards of the environmental laws.
Owner:INST NUCLEAR ENERGY RES ROCAEC
Method of recycling spent nuclear fuel
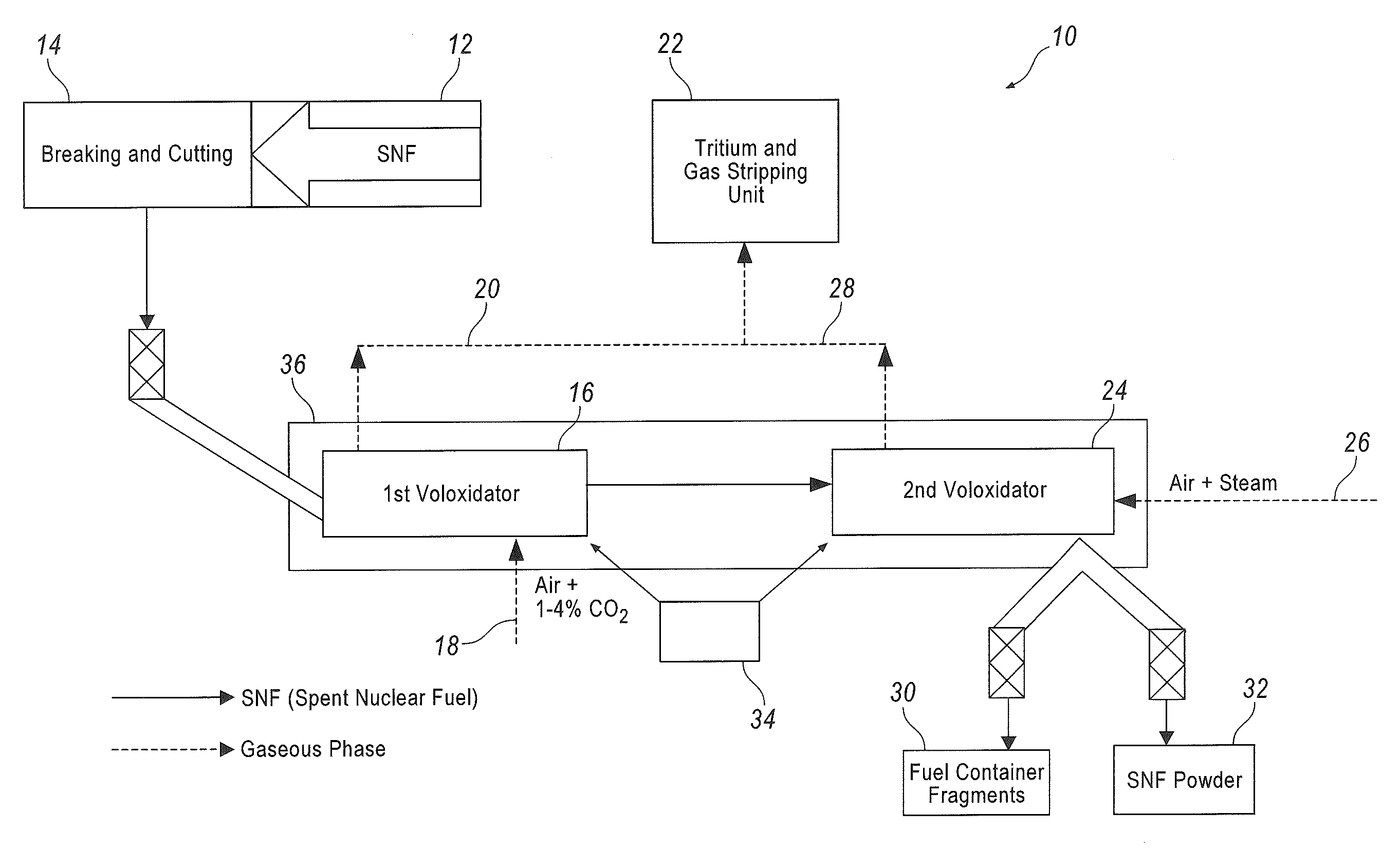
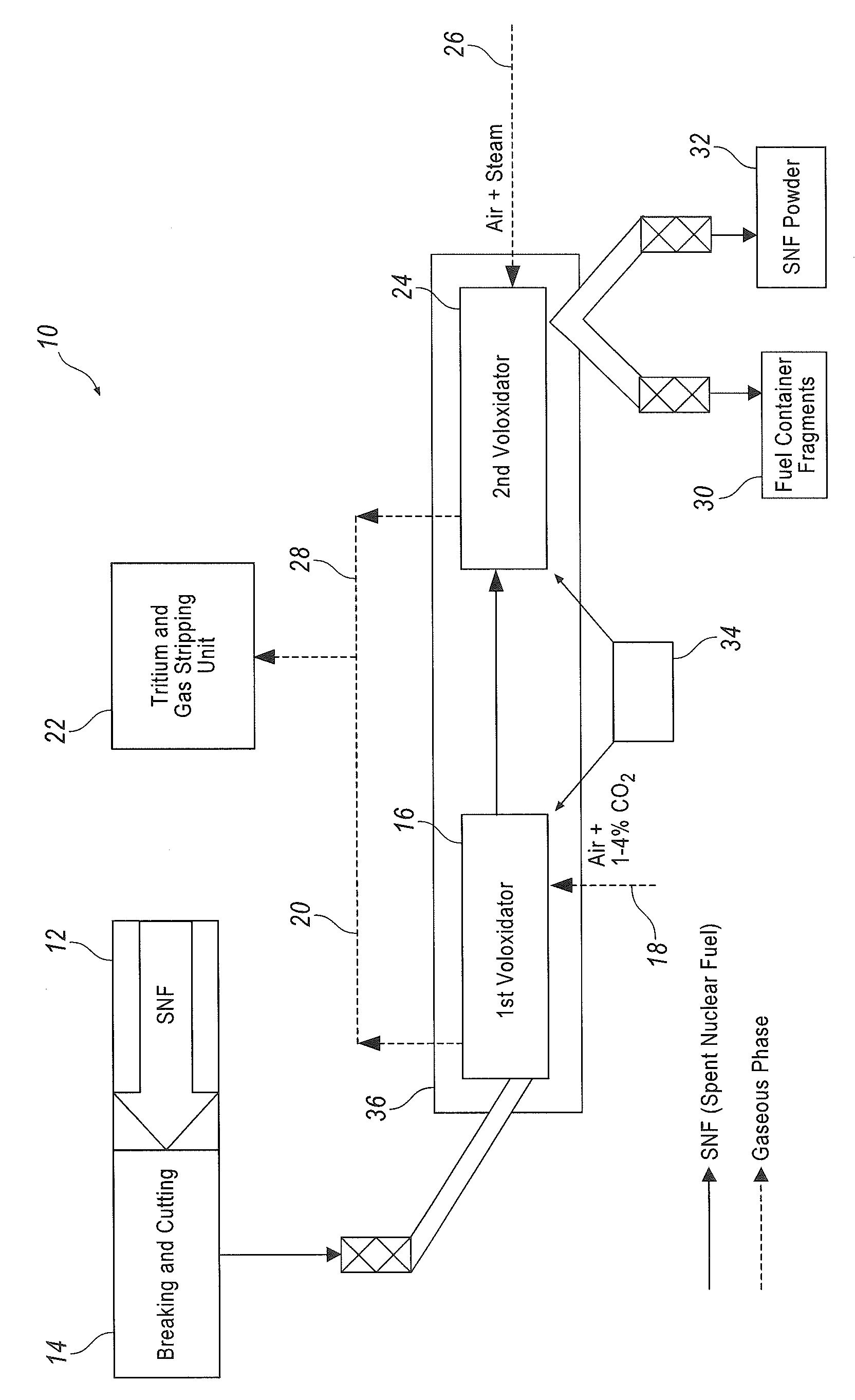
InactiveUS9147502B2Improve completenessNuclear fuel reprocessingNuclear energy generationBiological activationOxygen
The method concerns processing irradiated (spent) nuclear fuel (SNF), it is primarily aimed at isolating and trapping tritium, and can be used in nuclear power industry for treating SNF. This method provides for a two-phase voloxidation of a reaction mass using gas-air mixture, the reaction mass including fragmented uranium dioxide SNF elements with containers. The first phase is carried out at 400-650° C. in the presence of air and additional carbon dioxide. The second phase is carried out at 350-450° C. using a stream of an air-vapor mixture that can be oxygen-enriched. Both phases are carried out with a repeated mechanical activation of the reaction mass. Provided in the course of the voloxidation is the gas replacement at the hour rate of about 10-50 fold the reaction chamber gas volume. Before being introduced into the reaction chamber, the gas is preheated up to the chamber internal temperature.
Owner:FEDERAL INITARY ENTERPRISE MINING & CHEM COMBINE
Process for separating at least one first chemical element E1 from at least one second chemical element E2, involving the use of a medium comprising a specific molten salt
ActiveUS9677156B2Easy to separateReduce extractionNuclear fuel reprocessingNuclear energy generationLiquid stateChemical element
The invention pertains to a process for separating at least one first chemical element E1 from at least one second chemical element E2 coexisting in a mixture in the form of oxides, comprising the following steps:a) a step to solubilise a powder of one or more oxides of the said at least one first chemical element E1 and a powder of one or more oxides of the said at least one second chemical element E2 in a medium comprising at least one molten salt of formula MF—AlF3 wherein M is an alkaline element, after which there results after this step a mixture comprising the said molten salt, a fluoride of the said at least one first chemical elements E1 and a fluoride of the said at least one second chemical element E2;b) a step to contact the mixture resulting from step a) with a medium comprising a metal in the liquid state, the said metal being a reducing agent capable of predominantly reducing the said at least one first chemical element E1 relative to the said at least one second chemical element E2, after which there results after this step a two-phase medium comprising a first phase called metal phase comprising the said at least one first chemical element E1 in oxidation state 0, and a second phase called saline phase comprising the molten salt of above-mentioned formula MF—AlF3 and a fluoride of the said at least one second chemical element E2.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Method of recovering enriched radioactive technetium and system therefor
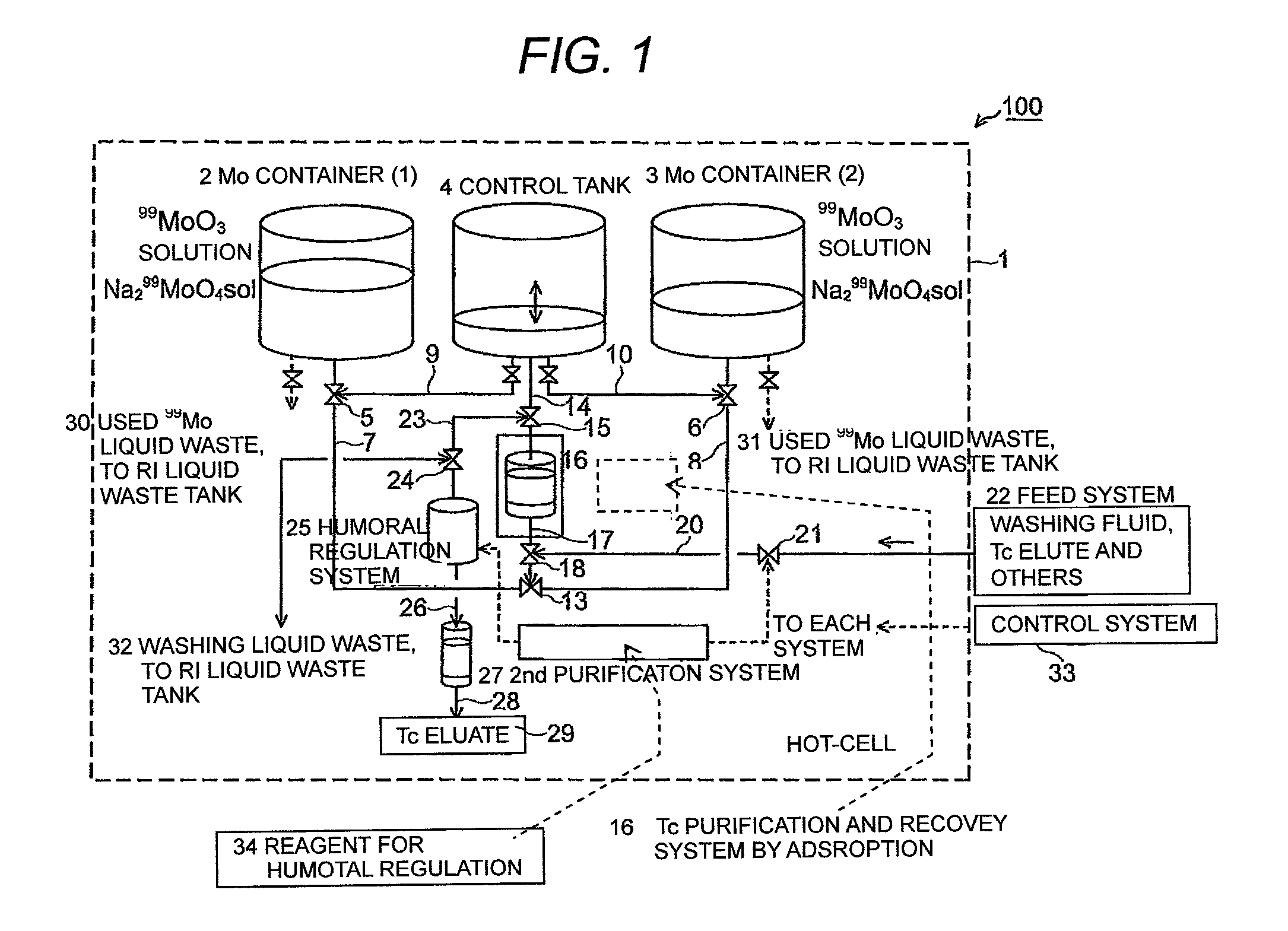
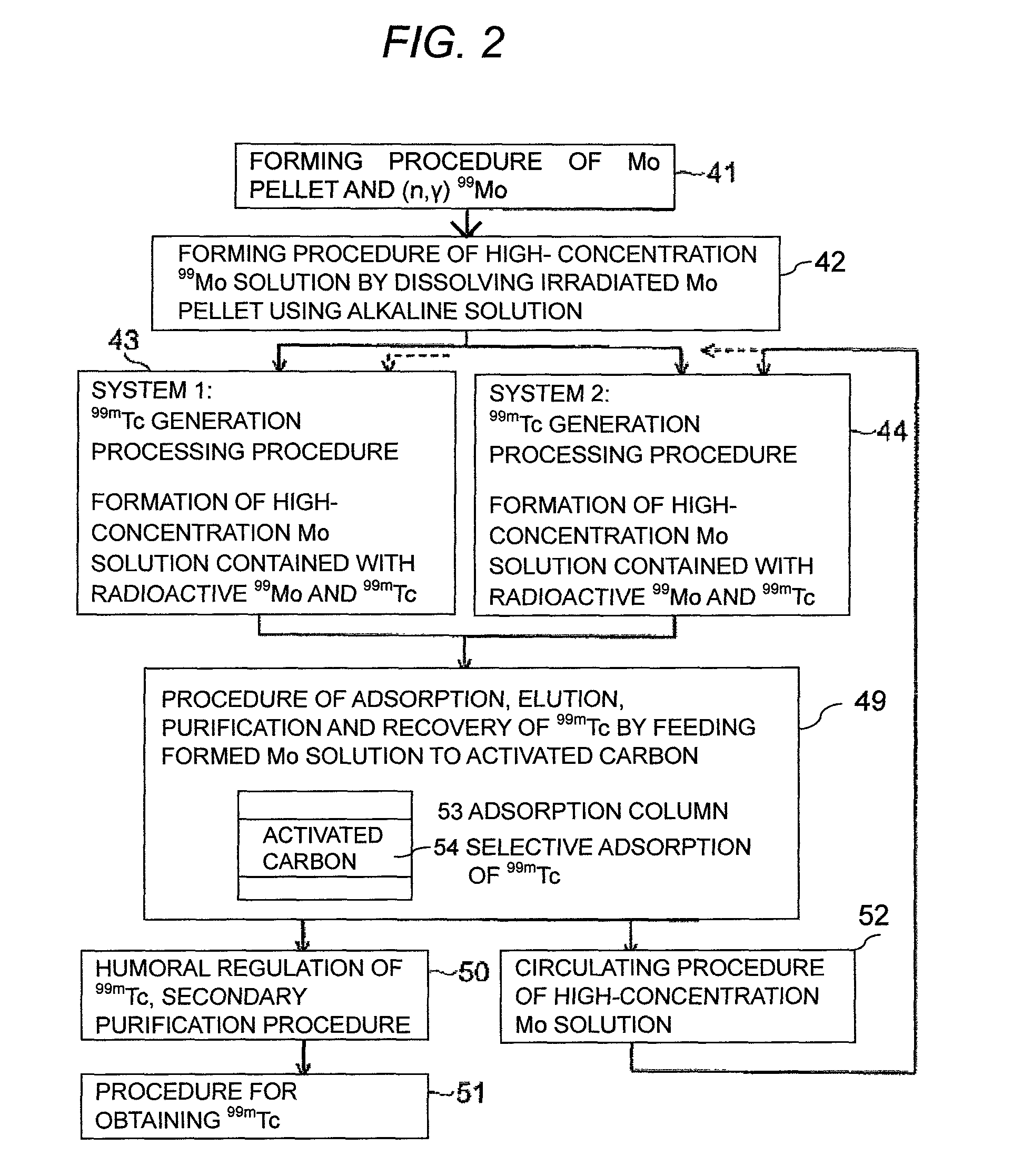
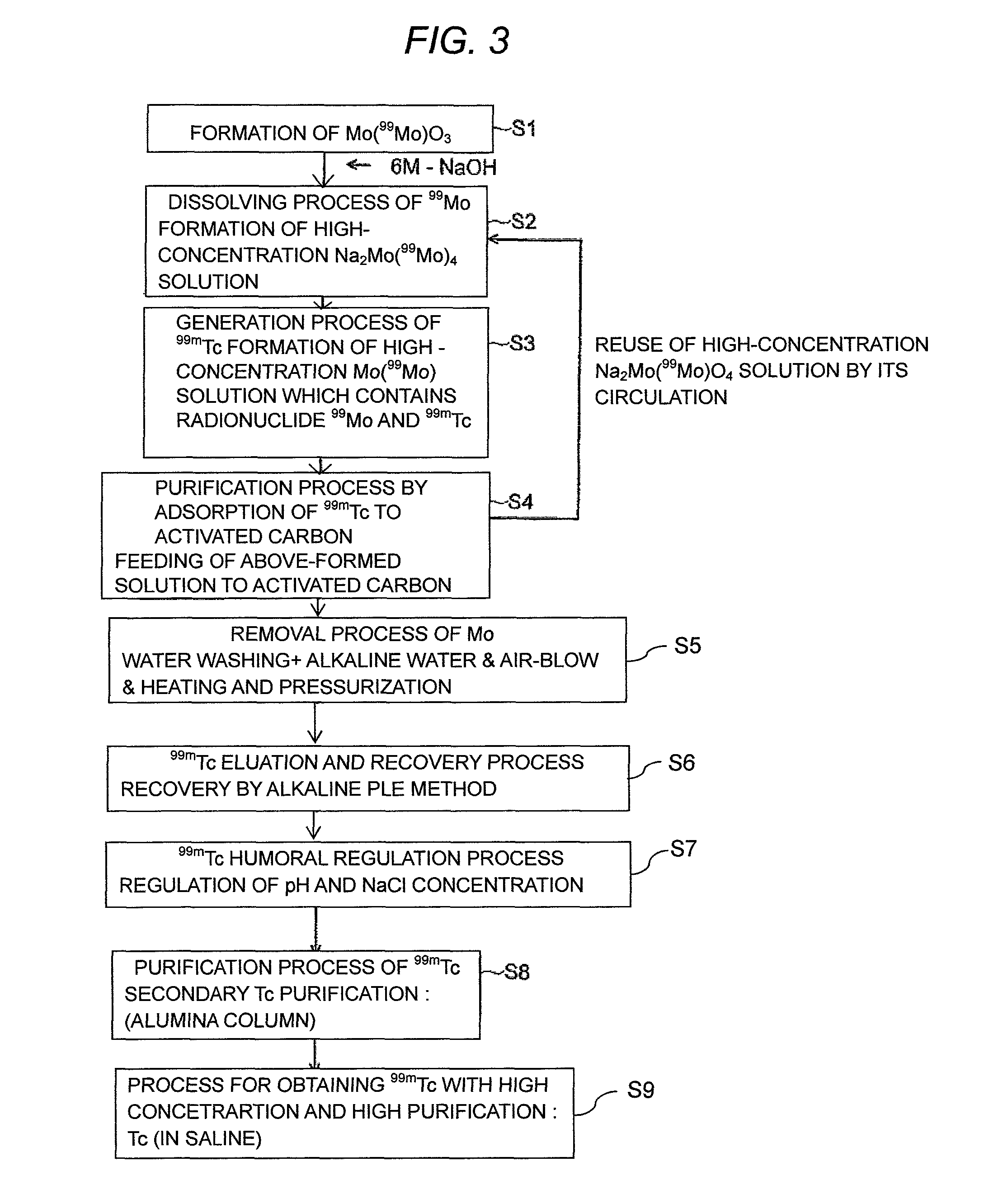
ActiveUS9236153B2Sufficient performanceHigh puritySpecific isotope recoveryNuclear fuel reprocessingHigh concentrationActivated carbon
To use 99mTc as a raw material for a radioactive medicine, a very small amount of 99mTc in the high concentration Mo(99Mo) solution is purified and recovered with high yield without contamination of 99Mo. 99mTc with high purity is recovered by forming a high concentration Mo(99Mo) solution which contains radionuclides 99Mo which is the parent nuclide of 99mTc used for the radioactive medicine and the raw material for its labeled compound, forming a high concentration Mo(99Mo) solution which contains radionuclides 99Mo and 99mTc by generating 99mTc to a radioactive-equilibrium state, getting 99mTc in the high concentration Mo(99Mo) solution adsorbed to activated carbon selectively by feeding the solution to an adsorption column which has activated carbon, and undergoing desorption and purification treatment of 99mTc with a desorbent from the activated carbon to which 99mTc is adsorbed.
Owner:SK KAKEN CO LTD
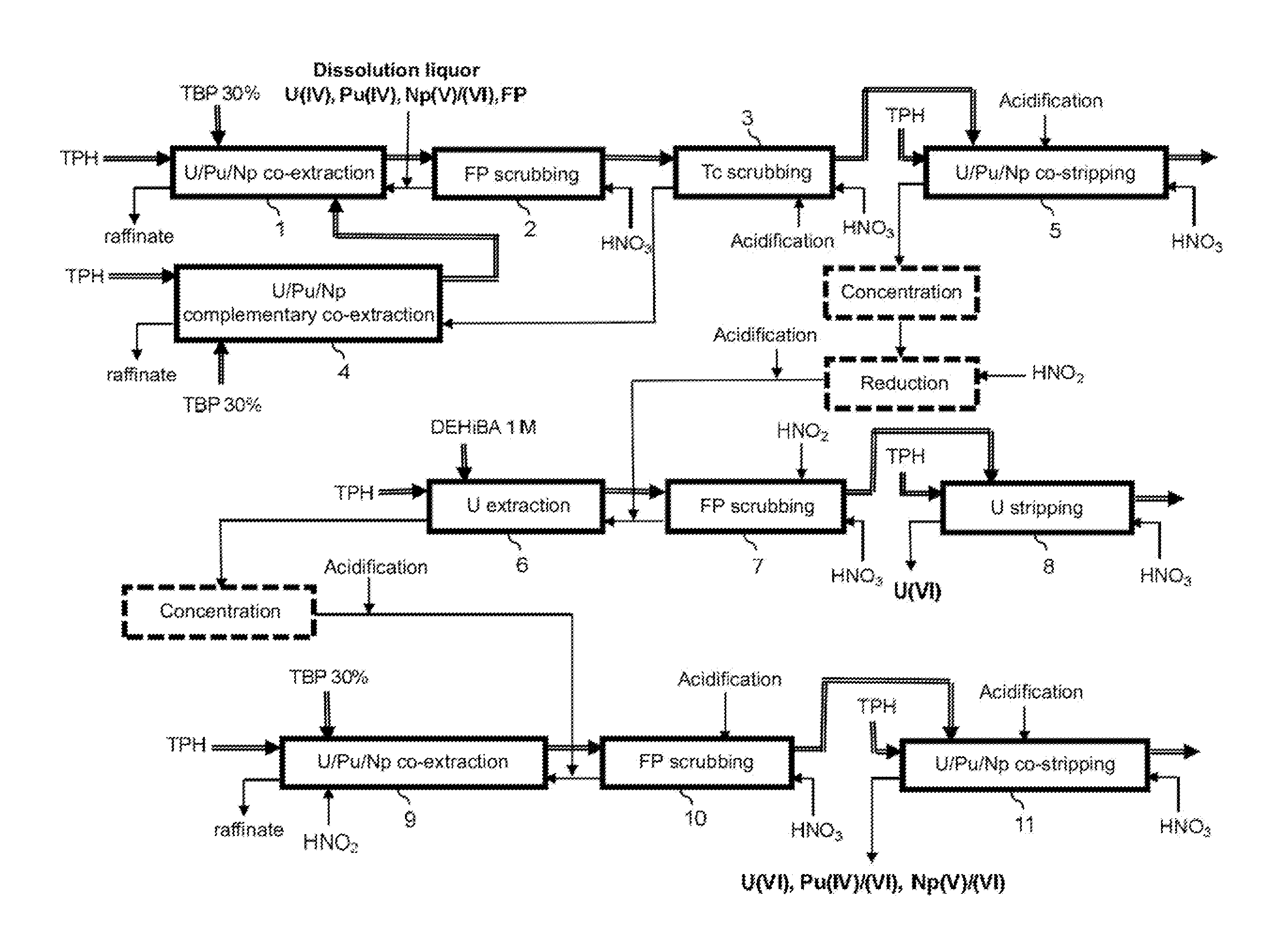
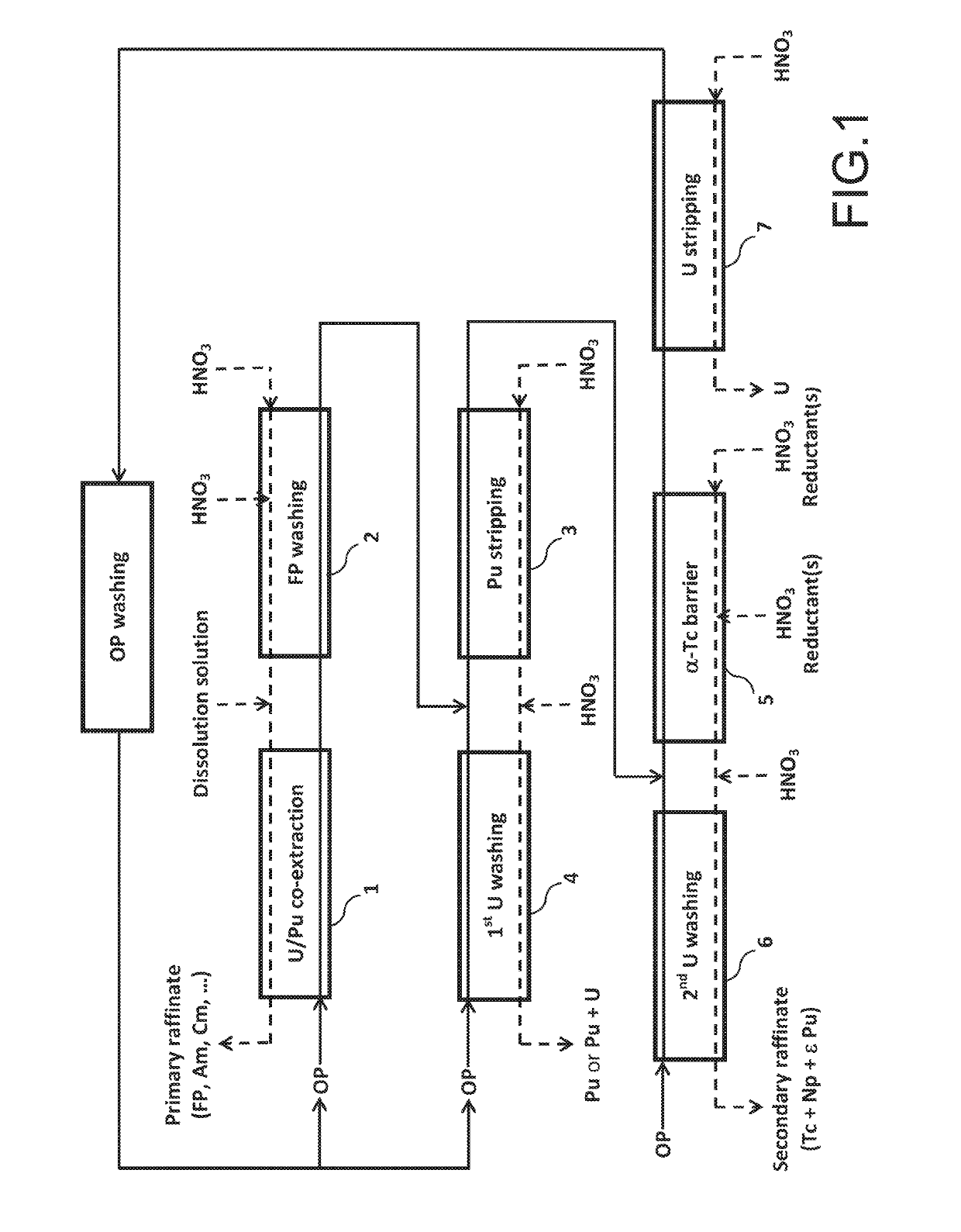
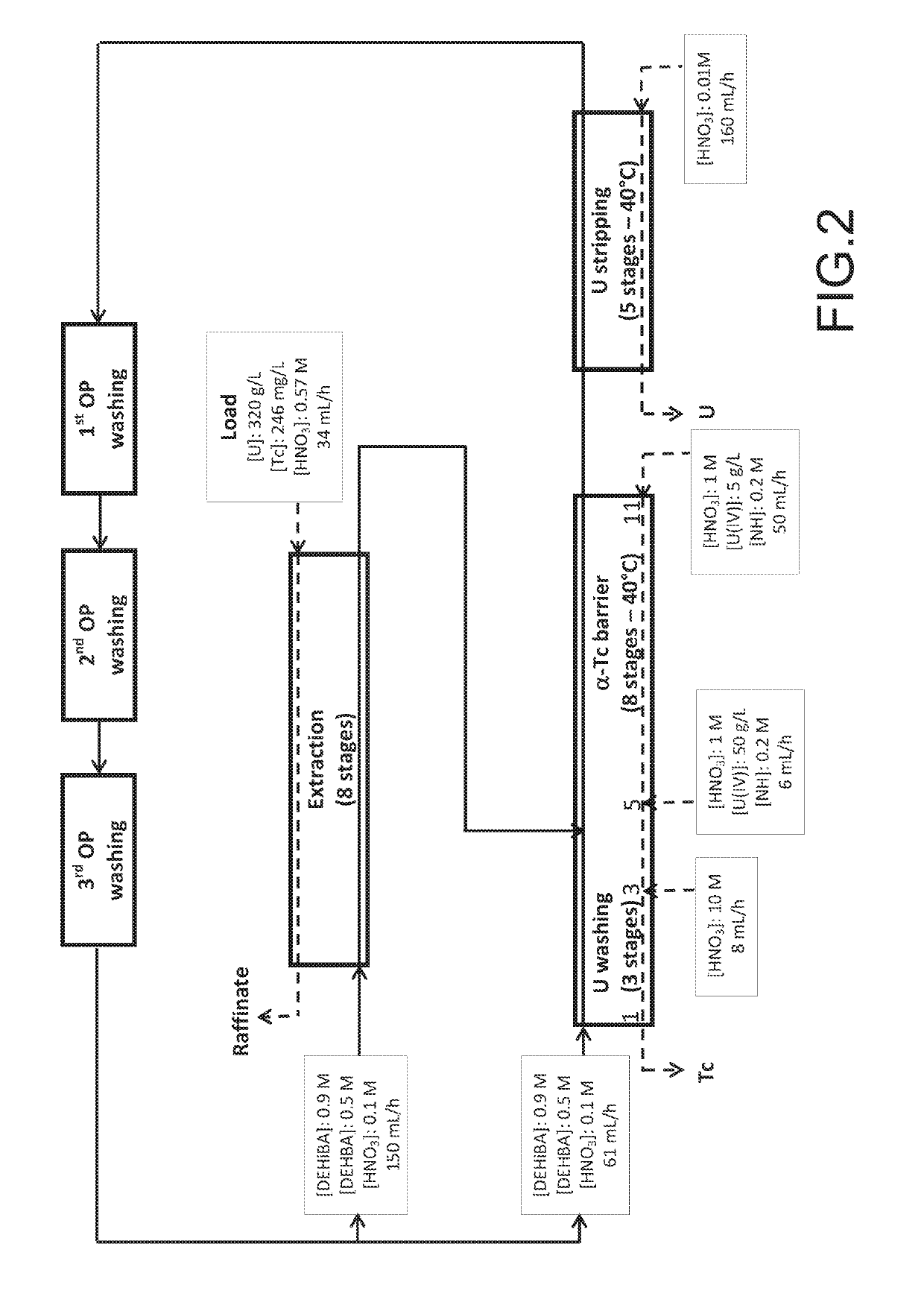
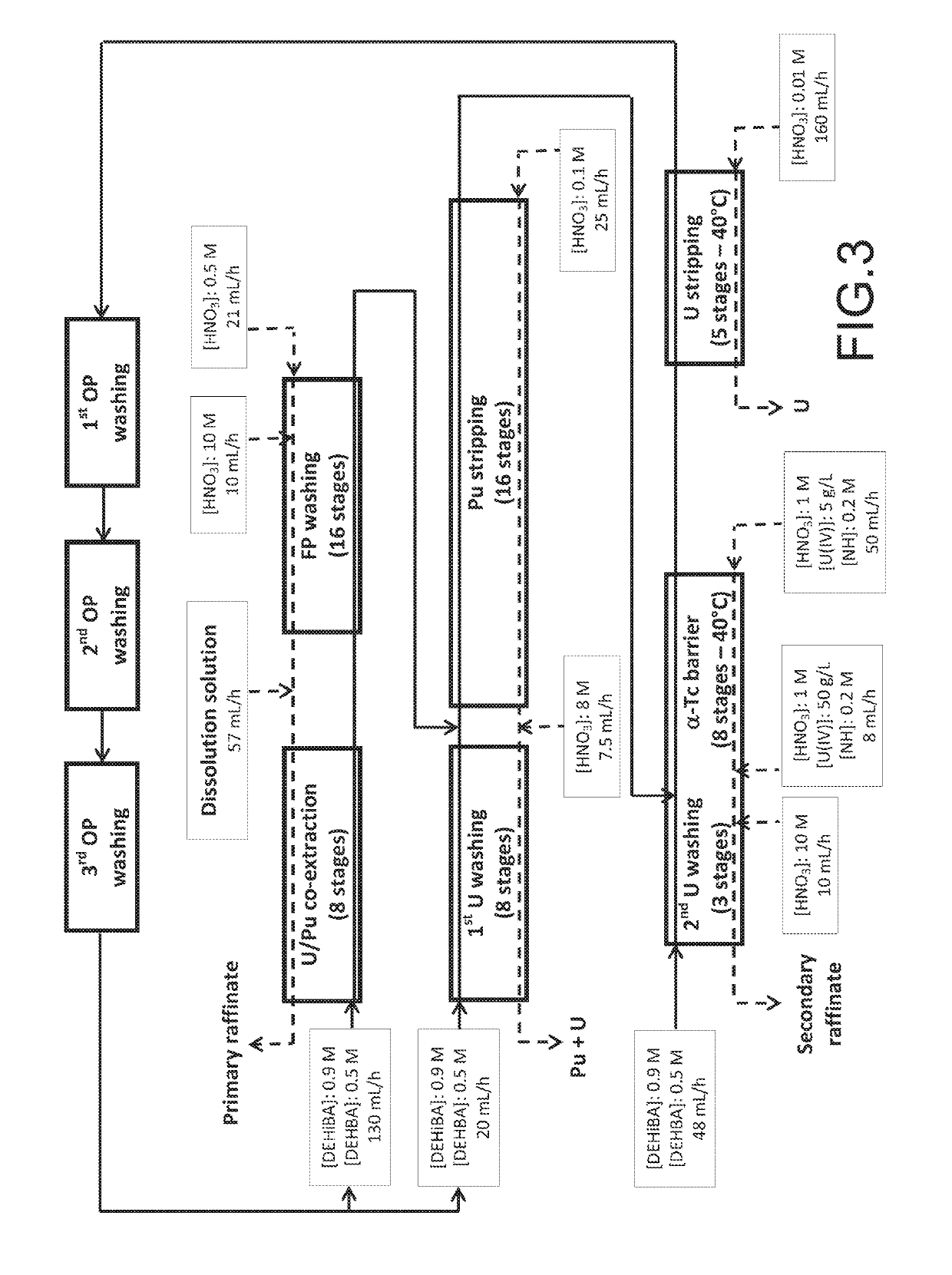
Method for the treatment of an aqueous nitric solution resulting from dissolving spent nuclear fuel, said method being performed in a single cycle and without requiring any operation involving reductive stripping of plutonium
ActiveUS10249396B2Easy to implementEliminate riskNuclear fuel reprocessingNuclear energy generationDissolutionAqueous solution
A method for the treatment of an aqueous solution resulting from the dissolution of a spent nuclear fuel in nitric acid, allowing the uranium and plutonium contained in the solution to be extracted, separated and decontaminated in a single cycle, without requiring any operation involving a reductive stripping of plutonium. Applications for the method include the processing of uranium-based and / or plutonium-based spent nuclear fuels.
Owner:ORANO RECYCLAGE +1
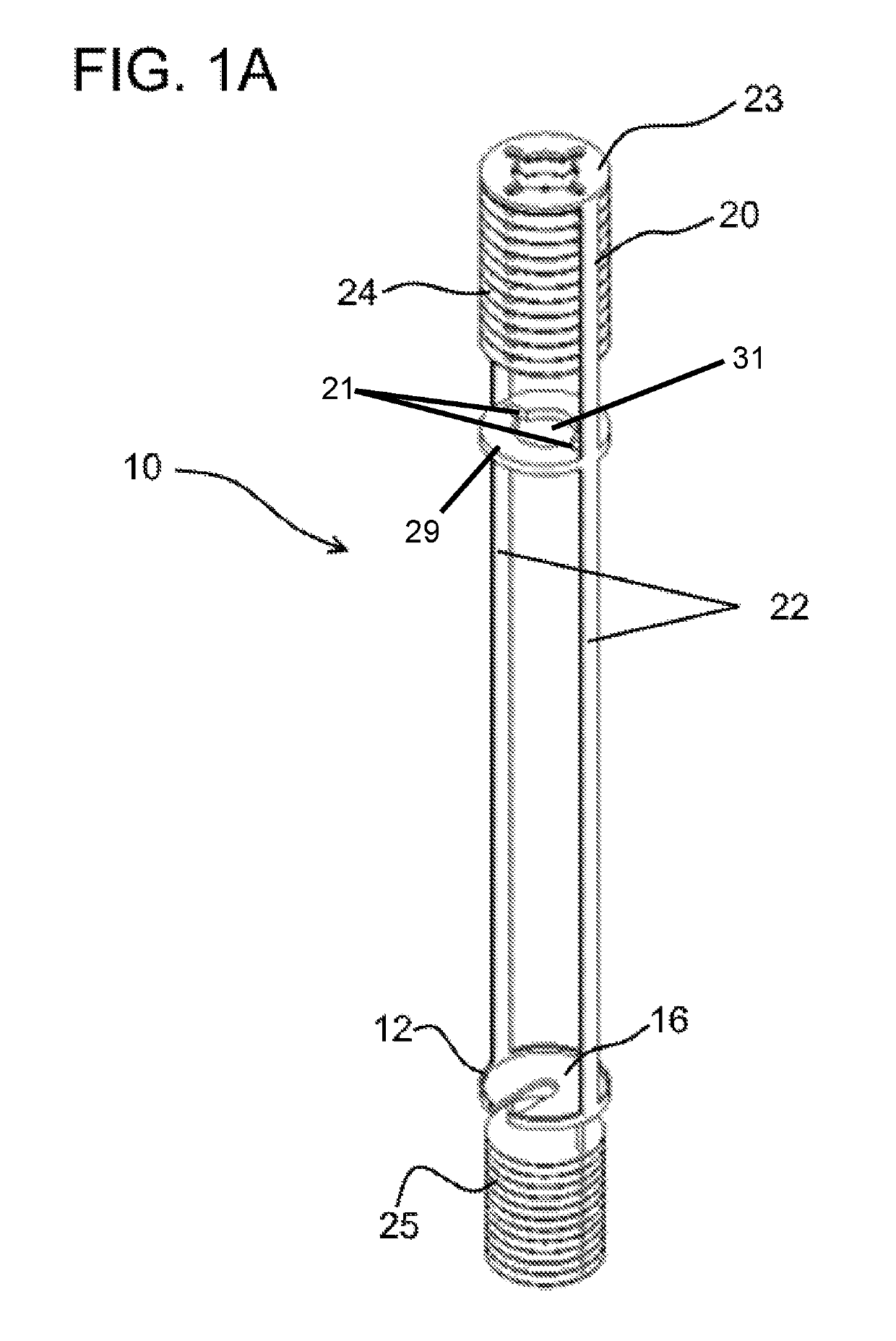
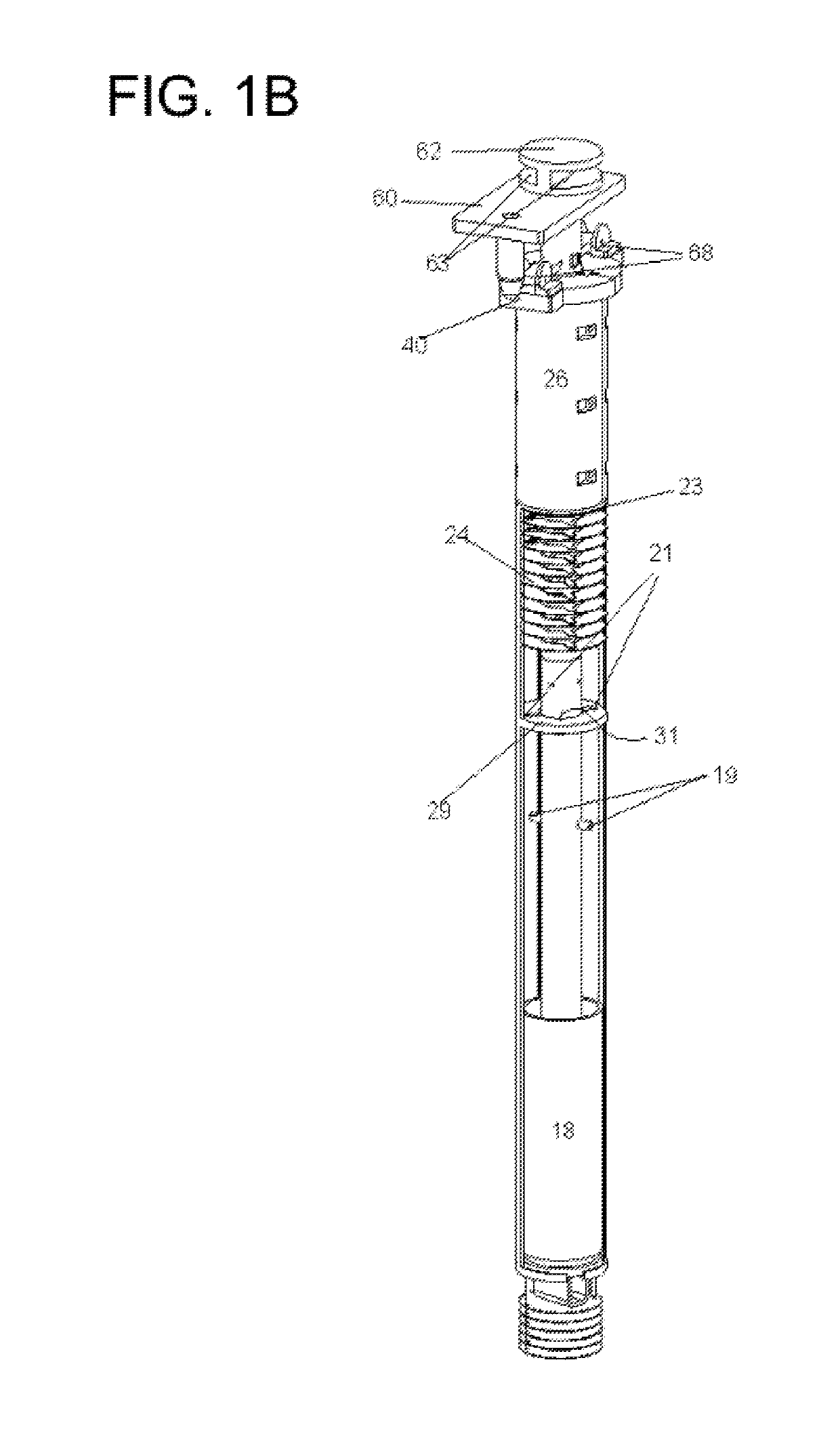
Integral U/TRU recovery cathode system for electrorefining used nuclear fuel, method for electrorefining and harvesting metal from used nuclear fuel
ActiveUS10301729B2Minimizing metal loss to electrolyteMinimize neutron levelNuclear fuel reprocessingNuclear energy generationElectrolysisMolten salt
The invention provides a system for collecting metal in an electrorefining process, the system having a hollow cathode; and a container defining an upwardly extending surface adapted to be received by the hollow cathode. An embodiment of the invention provides for metal reduction to occur on laterally facing and medially facing surfaces of the cathode such that electrolyte resides between surfaces of the cathode. Also provided is a metal electrorefining process having the steps of subjecting molten salt containing metal moieties to electrolysis wherein reduced metal accumulates in a cathode-cup construct in a first position; raising the construct to a second position above the molten salt while subjecting the construct to heat from the molten salt; withdrawing the cathode from the construct into a vestibule to the electrorefiner to a third position; and removing the cathode and cup from the electrorefiner to a fourth position.
Owner:UCHICAGO ARGONNE LLC
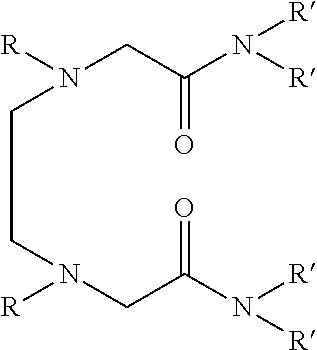
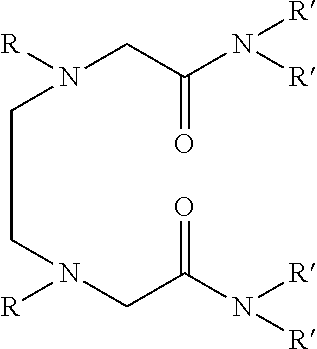
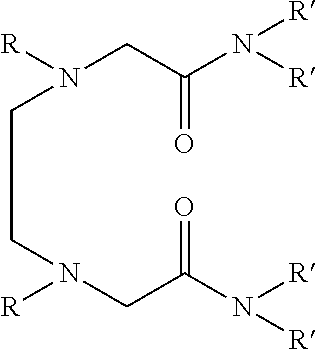
Dialkyldiaza-tetraalkyloctane diamide derivatives useful for the separation of trivalent actinides from lanthanides and process for the preparation thereof
The novel lipophilic metal extractants of the class dialkyldiaza-tetraalkyloctanediamide (DADA) useful to selectively separate trivalent americium (sup. 241 Am) from trivalent lanthanides are represented by the formula 1:Wherein R is a C1 to C5 normal alkyl and R′ is a C4 to C8 normal and branched alkyl group. The compounds are synthesized at high yield and purity by the reaction of corresponding N,N′-dialkylethylenediamine and N,N-dialkyl-2-chloroacetamide. The separation is achieved by utilizing the soft-soft interaction between the trivalent actinides and ‘N’ atoms of the extractant. Both soft donor “n” and hard donor ‘O’ sites are incorporated in the molecule for better extraction of trivalent actinides over trivalent lanthanides. Thus, this molecule can be used as selective extractant to separate trivalent actinides from trivalent lanthanides.
Owner:SEC DEPT OF ATOMIC ENERGY
Increase in the separation factor between americium and curium and/or between lanthanides in a liquid-liquid extraction operation
ActiveUS9284620B2Easy to separateNuclear fuel reprocessingNuclear energy generationSeparation factorRare earth
A method using diglycolamide for increasing the separation factor between americium and curium and / or between lanthanides during an extraction operation. The operation comprising putting an acid aqueous phase, in which are found the americium, curium and / or lanthanides, in contact with an organic phase non-miscible with water, containing at least one extractant in an organic diluent. The aqueous and organic phases are then separated, and the diglycolamide is added to the aqueous phase. This method can be used for processing and recycling irradiated nuclear fuels, in particular for selectively recovering americium from high activity aqueous solutions such as raffinates stemming from the processing of irradiated nuclear fuels with a PUREX or COEX™ method; processing of rare earth ores of the monazite, xenotime or bastnaesite type, in order to facilitate separation of <<lightweight>> rare earths from <<heavy>> rare earths and of yttrium, or that of two rare earths with adjacent or close atomic numbers.
Owner:ORANO RECYCLAGE +1
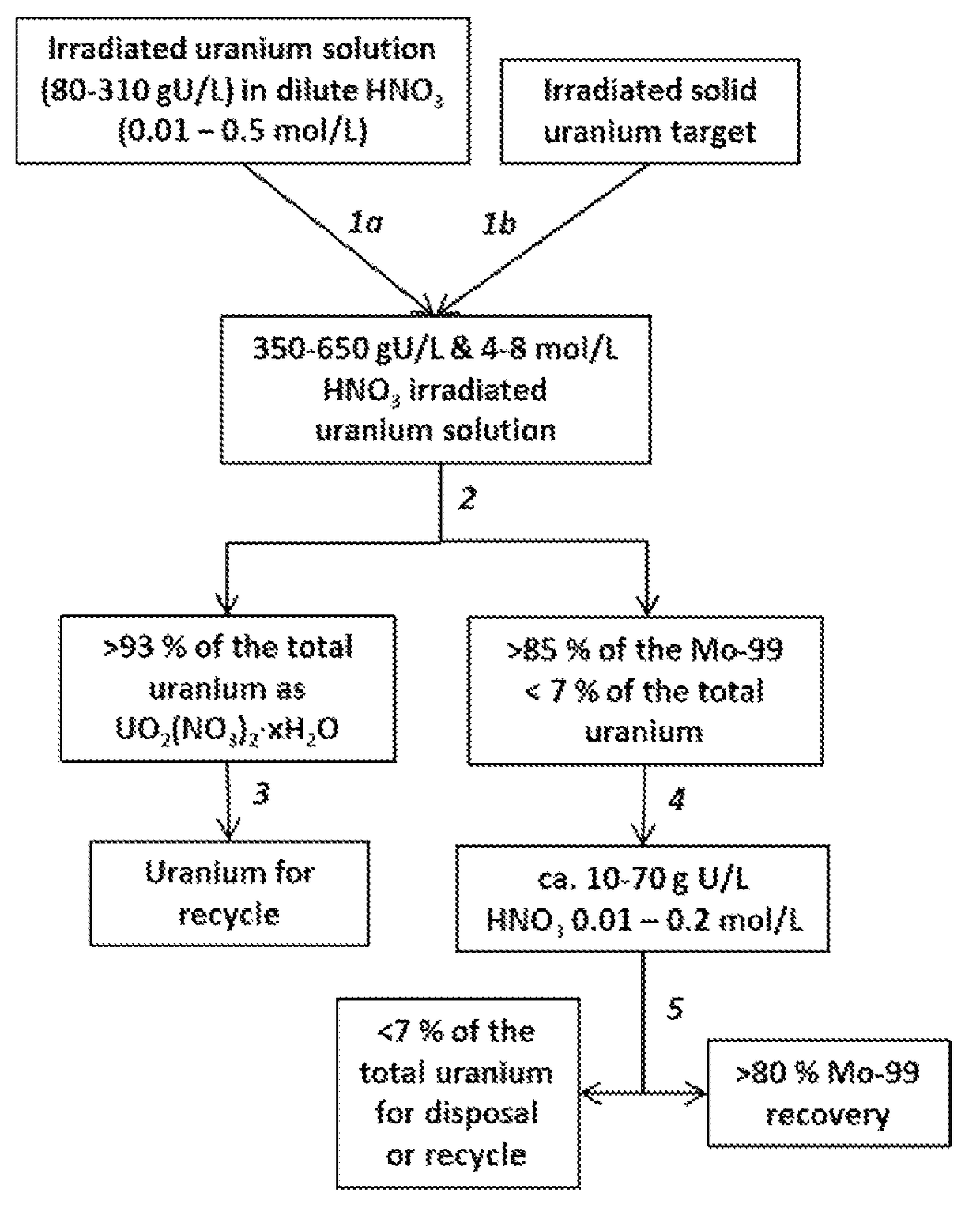
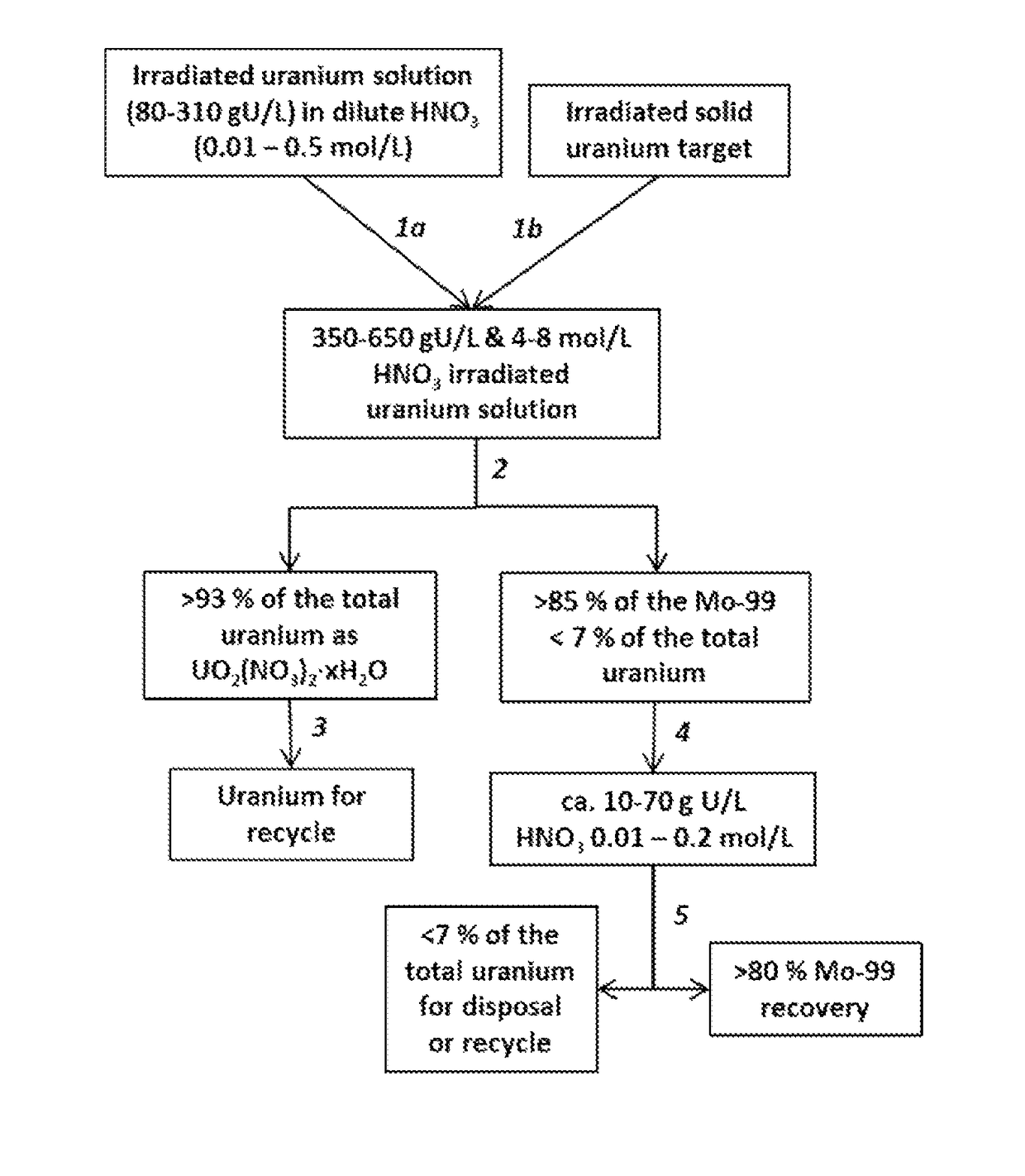
Recovering and recycling uranium used for production of molybdenum-99
A processes for recycling uranium that has been used for the production of molybdenum-99 involves irradiating a solution of uranium suitable for forming fission products including molybdenum-99, conditioning the irradiated solution to one suitable for inducing the formation of crystals of uranyl nitrate hydrates, then forming the crystals and a supernatant and then separating the crystals from the supernatant, thus using the crystals as a source of uranium for recycle. Molybdenum-99 is recovered from the supernatant using an adsorbent such as alumina. Another process involves irradiation of a solid target comprising uranium, forming an acidic solution from the irradiated target suitable for inducing the formation of crystals of uranyl nitrate hydrates, then forming the crystals and a supernatant and then separating the crystals from the supernatant, thus using the crystals as a source of uranium for recycle. Molybdenum-99 is recovered from the supernatant using an adsorbent such as alumina.
Owner:TRIAD NAT SECURITY LLC
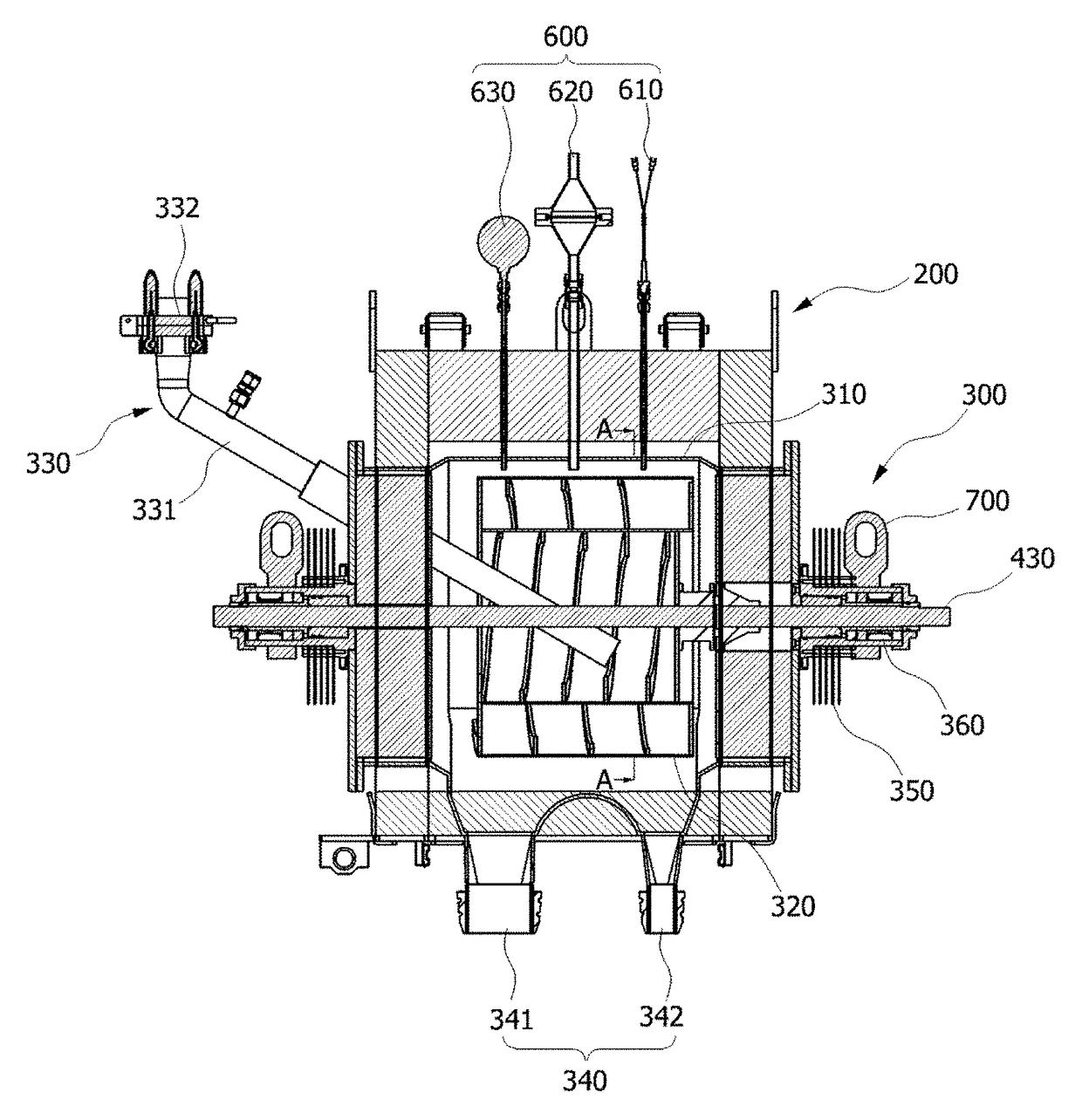
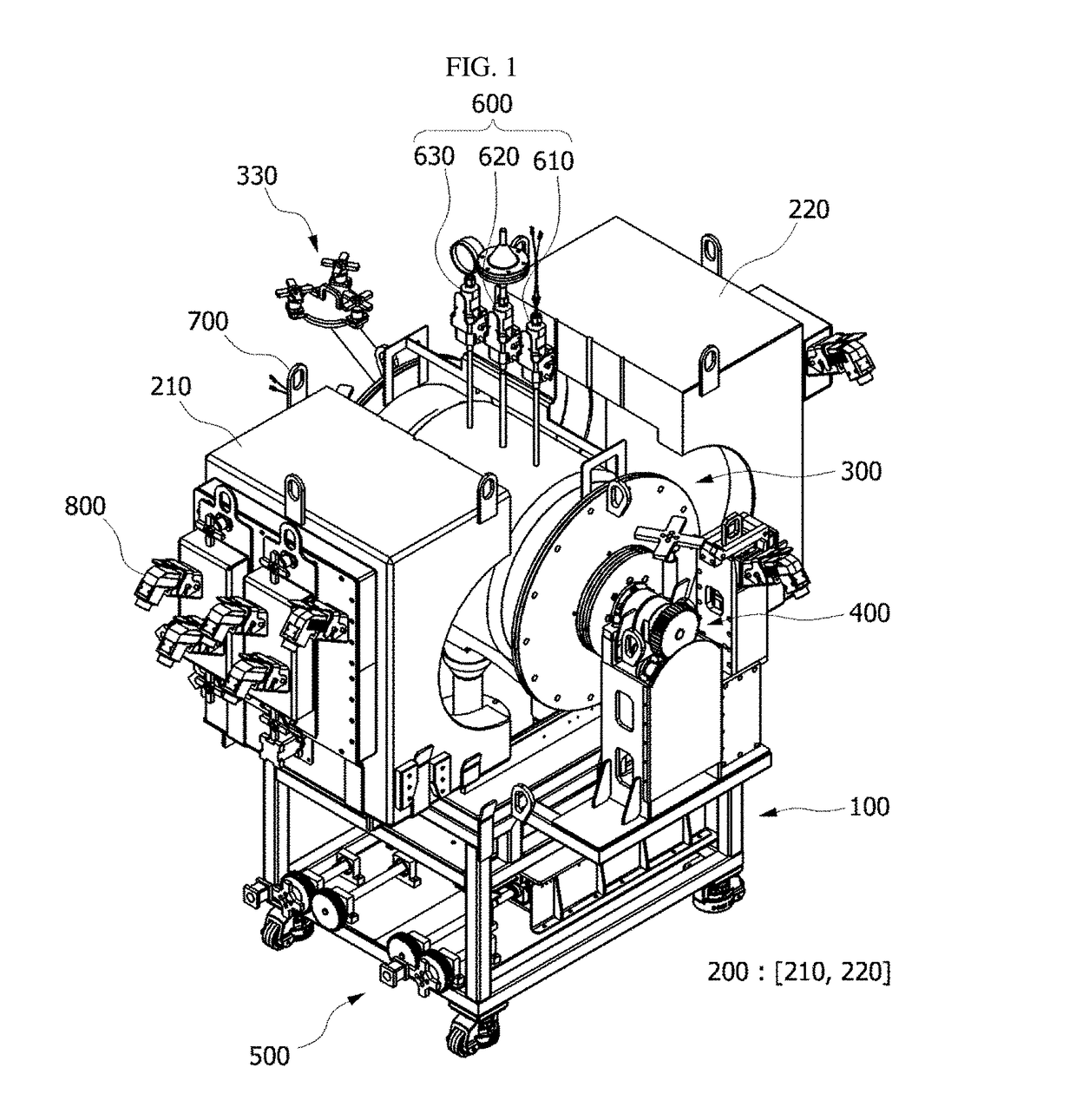
Voloxidizer with double reactor for spent fuel rods decladding and double reactor for use in the same
ActiveUS9711247B2Raise the ratioEasy to separateNuclear fuel reprocessingNuclear energy generationNuclear engineering
Owner:KOREA ATOMIC ENERGY RES INST
Use of certain chemical elements for inhibiting the formation of precipitates containing zirconium molybdate in an aqueous solution containing the element molybdenum and the element zirconium
ActiveUS8529769B2Rapid increase in solubilityLittle strengthNuclear fuel reprocessingSolvent extractionChemical elementTe element
A method inhibits the formation of zirconium molybdate precipitate in an aqueous solution containing the element molybdenum and the element zirconium by adding a chemical element selected from plutonium, tellurium, antimony and mixtures thereof with the aqueous solution. The method can be used for reprocessing used fuels with the element molybdenum and the element zirconium.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
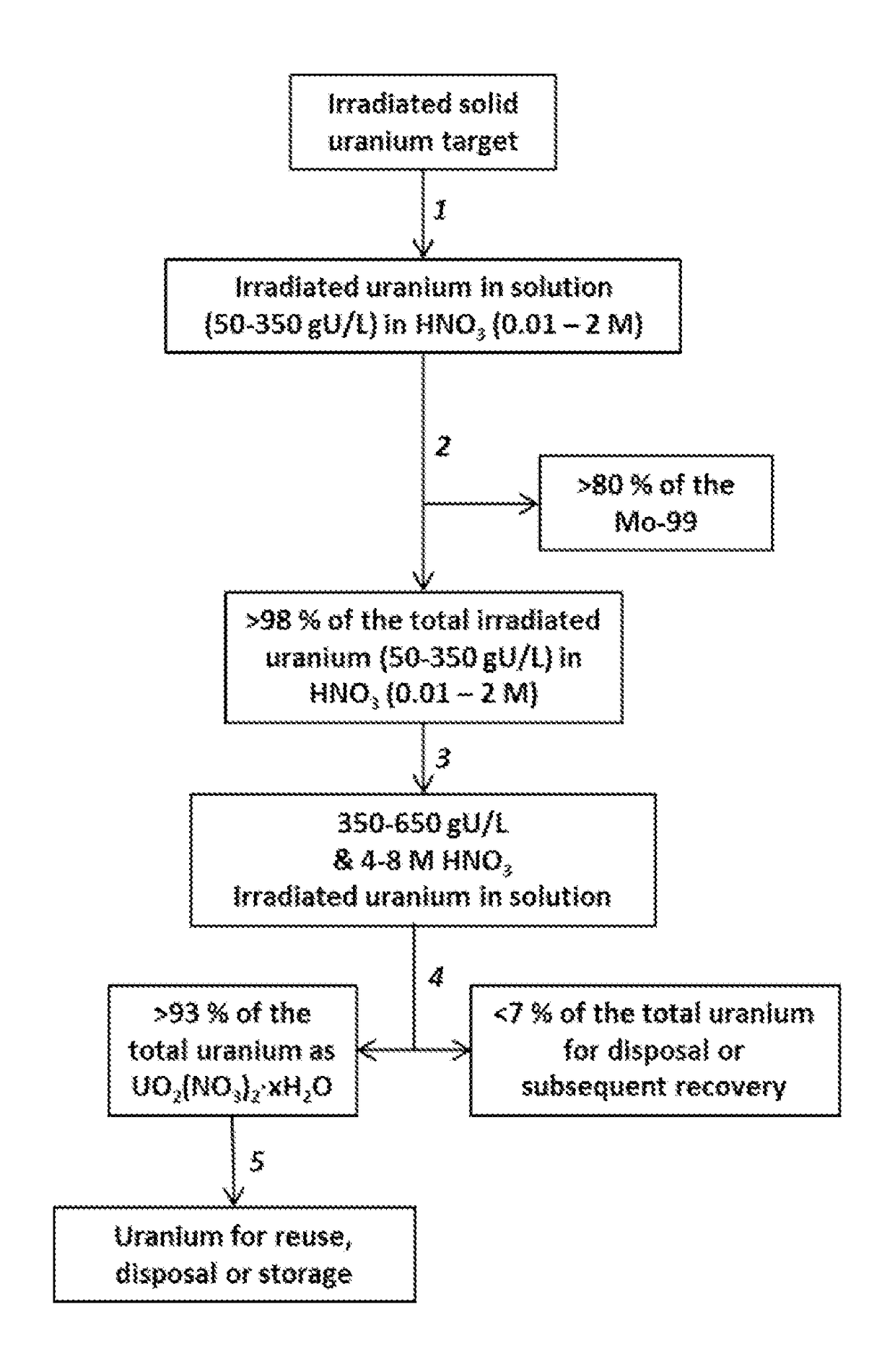
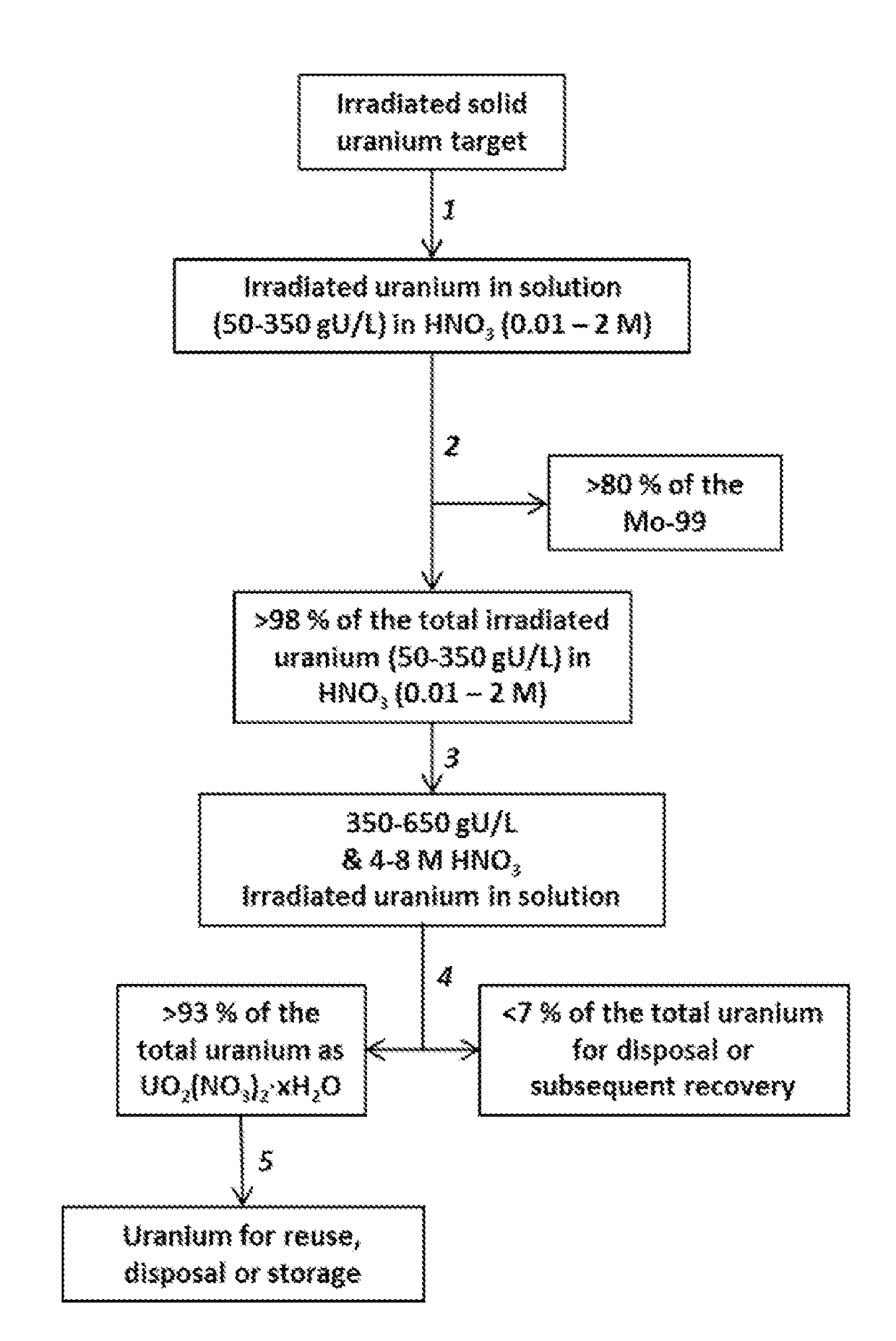
Recovery of uranium from an irradiated solid target after removal of molybdenum-99 produced from the irradiated target
A process for minimizing waste and maximizing utilization of uranium involves recovering uranium from an irradiated solid target after separating the medical isotope product, molybdenum-99, produced from the irradiated target. The process includes irradiating a solid target comprising uranium to produce fission products comprising molybdenum-99, and thereafter dissolving the target and conditioning the solution to prepare an aqueous nitric acid solution containing irradiated uranium. The acidic solution is then contacted with a solid sorbent whereby molybdenum-99 remains adsorbed to the sorbent for subsequent recovery. The uranium passes through the sorbent. The concentrations of acid and uranium are then adjusted to concentrations suitable for crystallization of uranyl nitrate hydrates. After inducing the crystallization, the uranyl nitrate hydrates are separated from a supernatant. The process results in the purification of uranyl nitrate hydrates from fission products and other contaminants. The uranium is therefore available for reuse, storage, or disposal.
Owner:TRIAD NAT SECURITY LLC
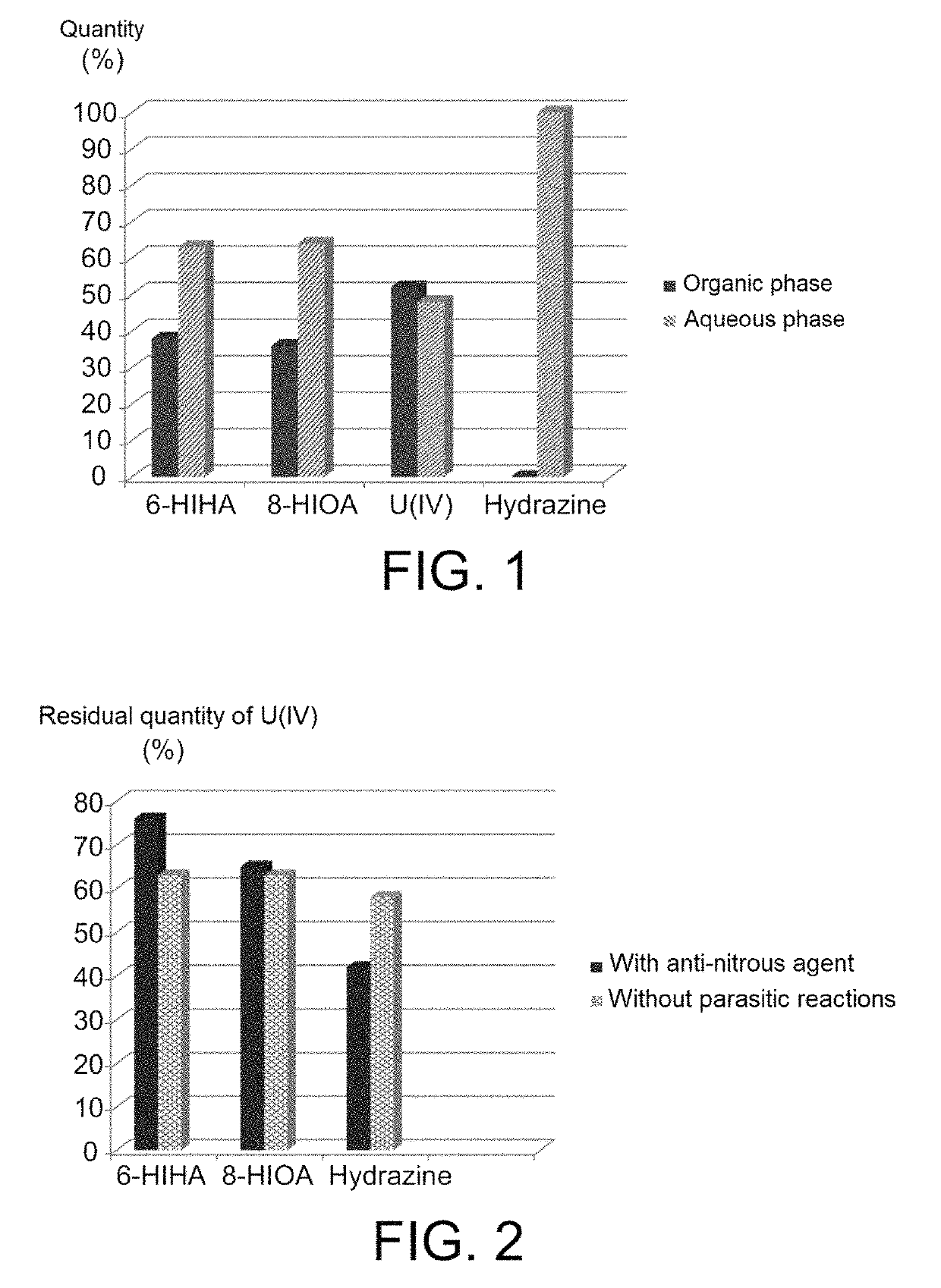
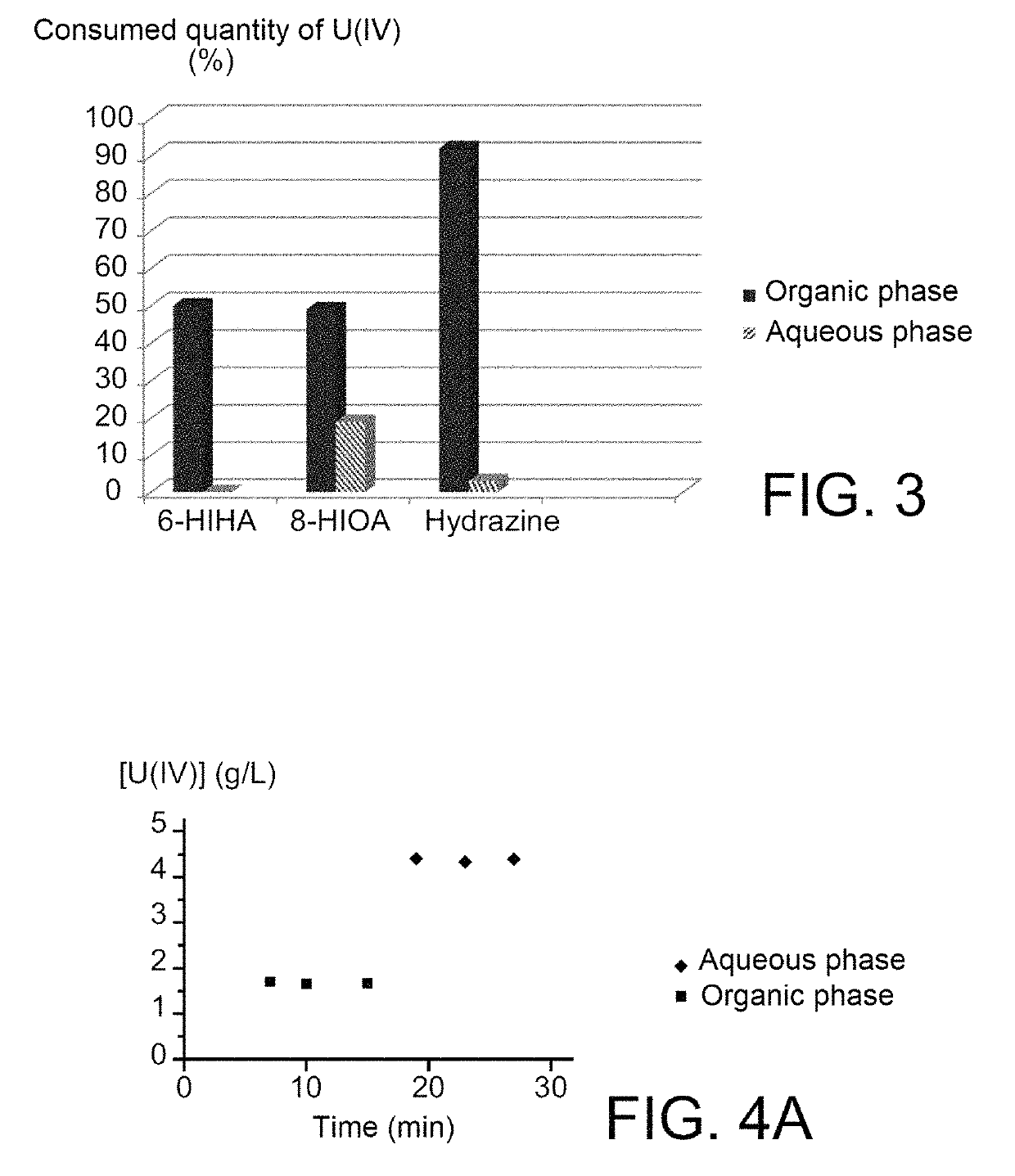
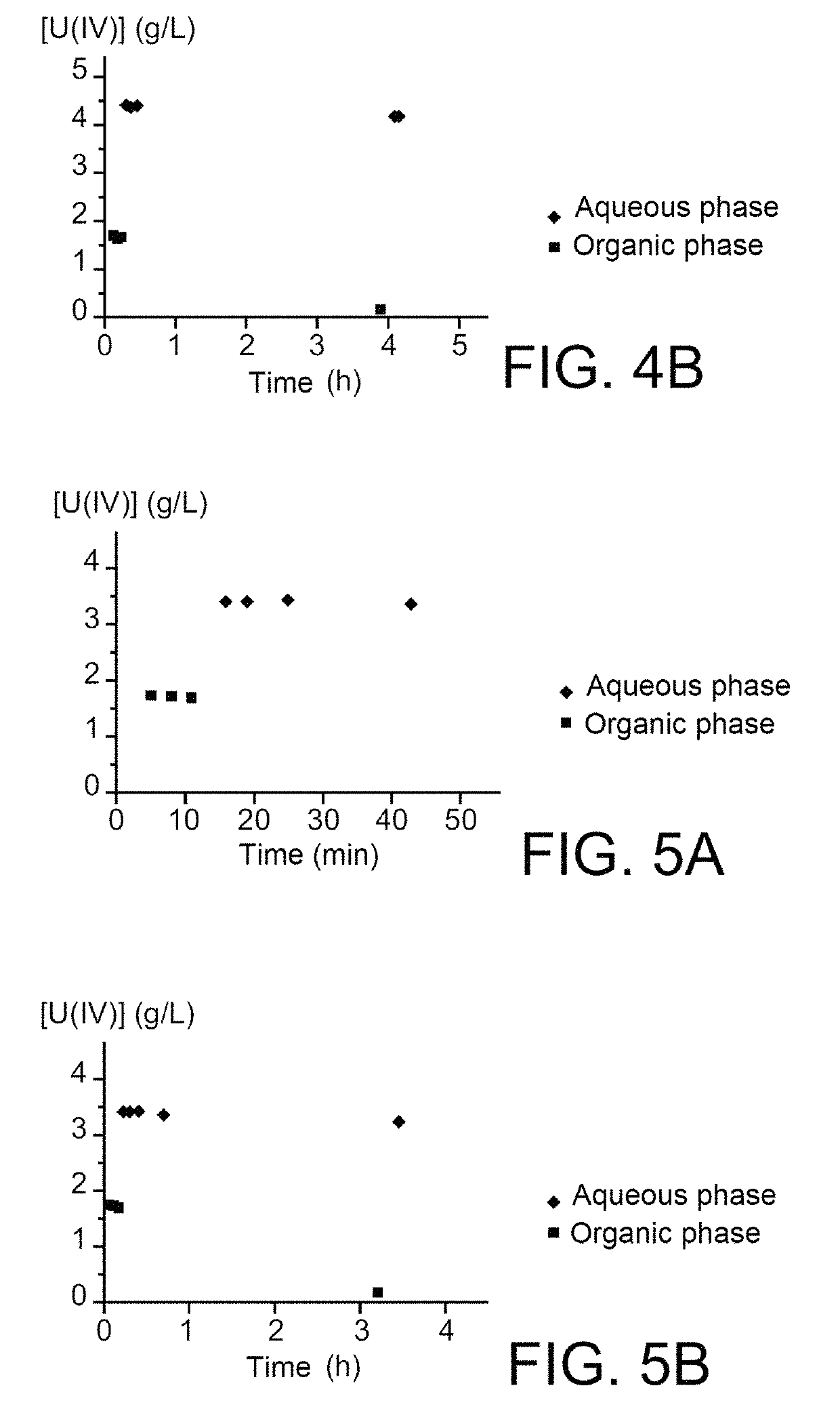
Use of hydroxyiminoalkanoic acids as anti-nitrous agents in operations of reductive stripping of plutonium
ActiveUS10354765B2Efficiently blocking the reoxidation of plutoniumStrong reduction in quantityNuclear fuel reprocessingNuclear energy generationProcessing plantsNuclear fuel
The use of hydroxyiminoalkanoic acids including at least four carbon atoms as anti-nitrous agents in operations of reductive stripping of plutonium. The invention may be useful in any method for processing spent nuclear fuels that includes one or more operations of reductive stripping of plutonium and, more particularly, in the PUREX method as implemented in modern nuclear fuel processing plants, as well as in processes derived therefrom.
Owner:ORANO RECYCLAGE +1
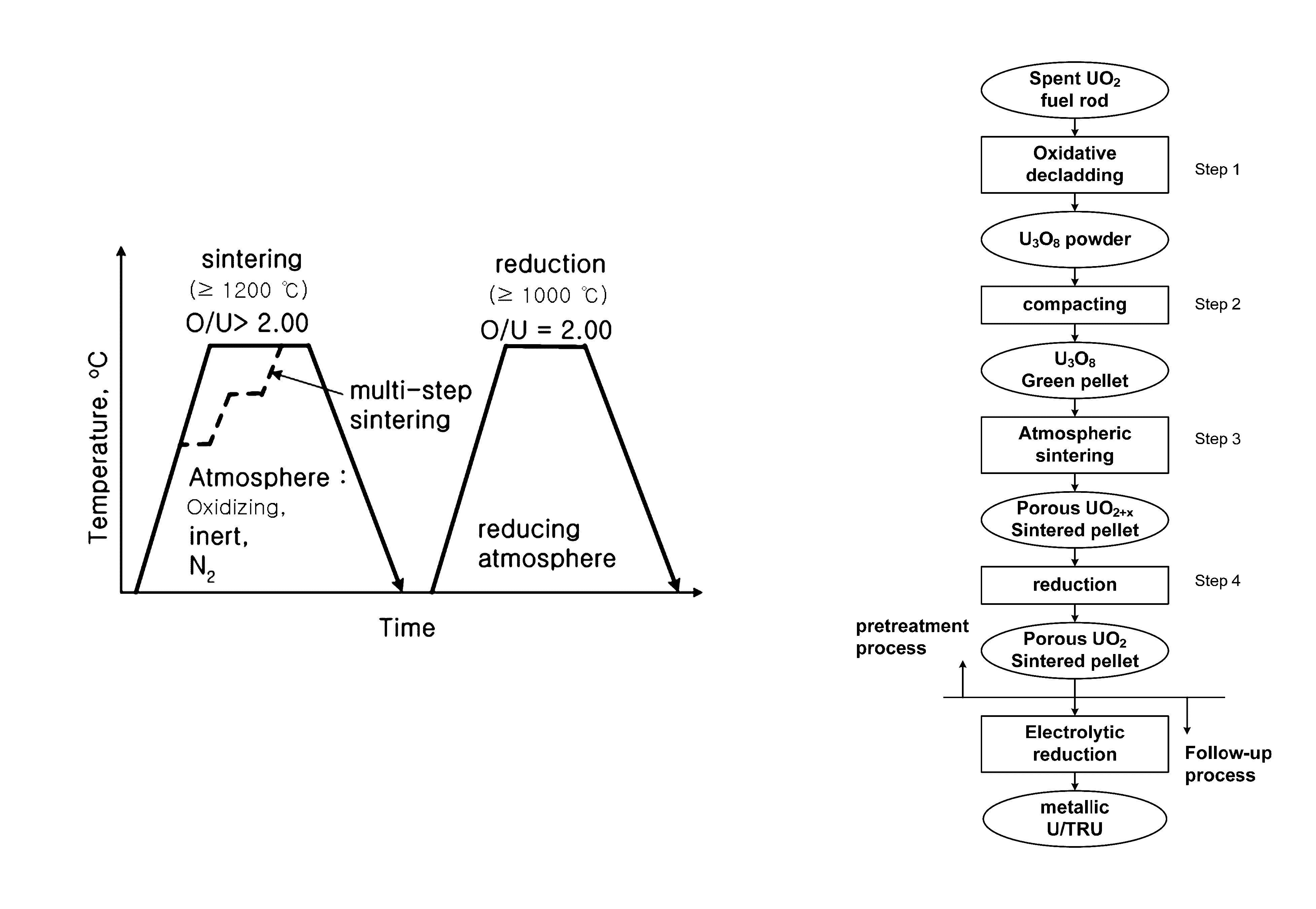
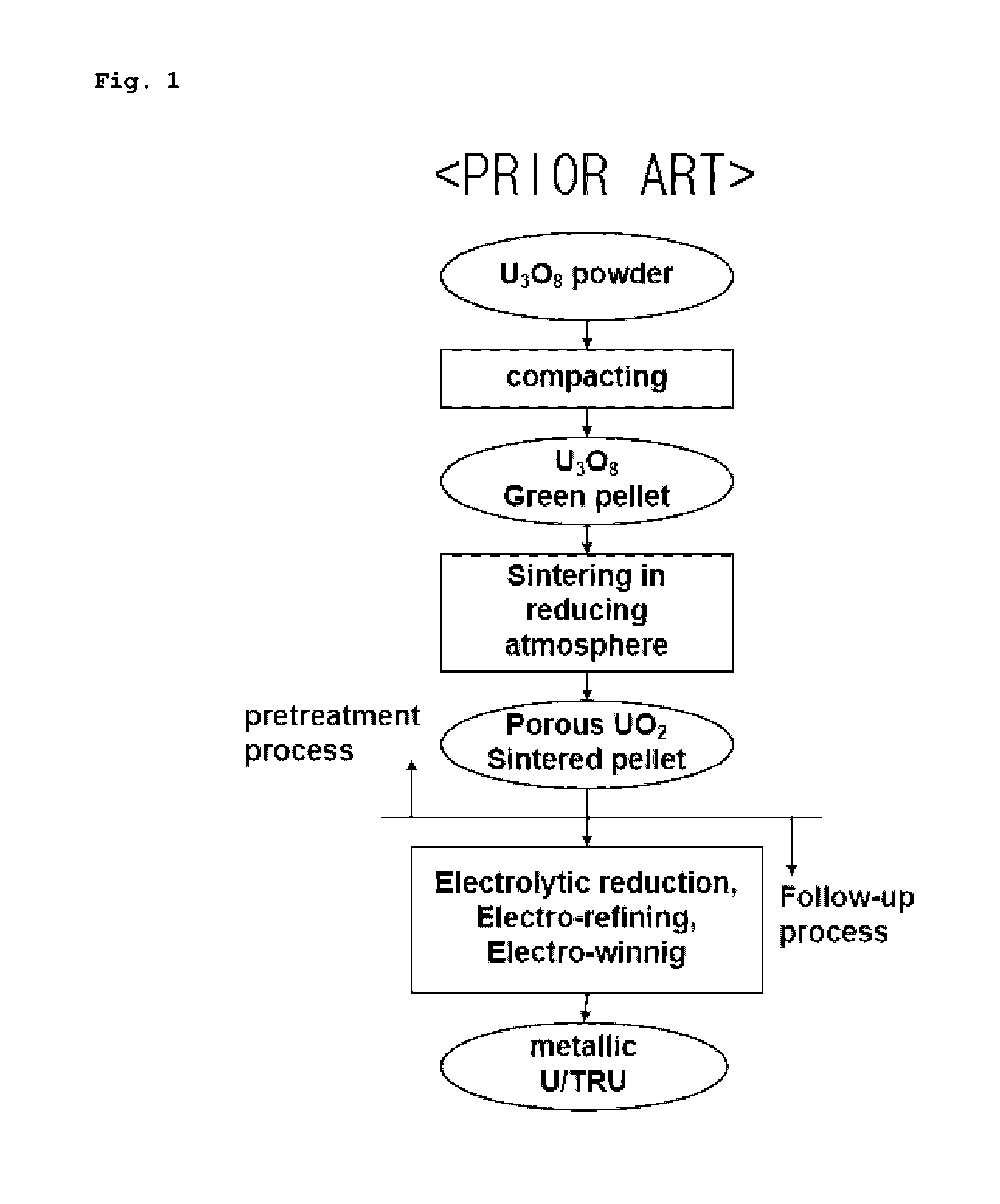
Porous UO2 sintered pellets and method for fabricating porous UO2 sintered pellets and electrolytic reduction using same
ActiveUS9558853B2Improve rigidityEasy to handleNuclear fuel reprocessingFuel elementsRoom temperatureReducing atmosphere
Owner:KOREA ATOMIC ENERGY RES INST +1
Process for preparing an oxyhalogenide and/or oxide of actinide(s) and/or of lanthanide(s) from a medium comprising at least one molten salt
ActiveUS9302918B2Easy to recycleNuclear fuel reprocessingRare earth metal chloridesLanthanideMolten salt
The invention relates to a process for manufacturing an oxychloride or oxide of actinide(s) and / or of lanthanide(s) from a chloride of actinide(s) and / or of lanthanide(s) present in a medium comprising at least one molten salt of chloride type comprising a step of bringing said chloride of actinide(s) and / or lanthanide(s) present in said medium comprising at least one molten salt of chloride type into contact with a wet inert gas.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Process for preparing a powder comprising a solid solution of uranium dioxide and of a dioxide of at least one other actinide and/or lanthanide element
A method for preparing a powder of a solid solution of dioxide of uranium and of at least one other actinide and / or lanthanide element comprising combusting a solution that comprises uranyl nitrate and at least one nitrate of the other actinide and / or lanthanide element and glycine, with the glycine being used in a predetermined amount so as to form, at the end of the combustion, the solid solution.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
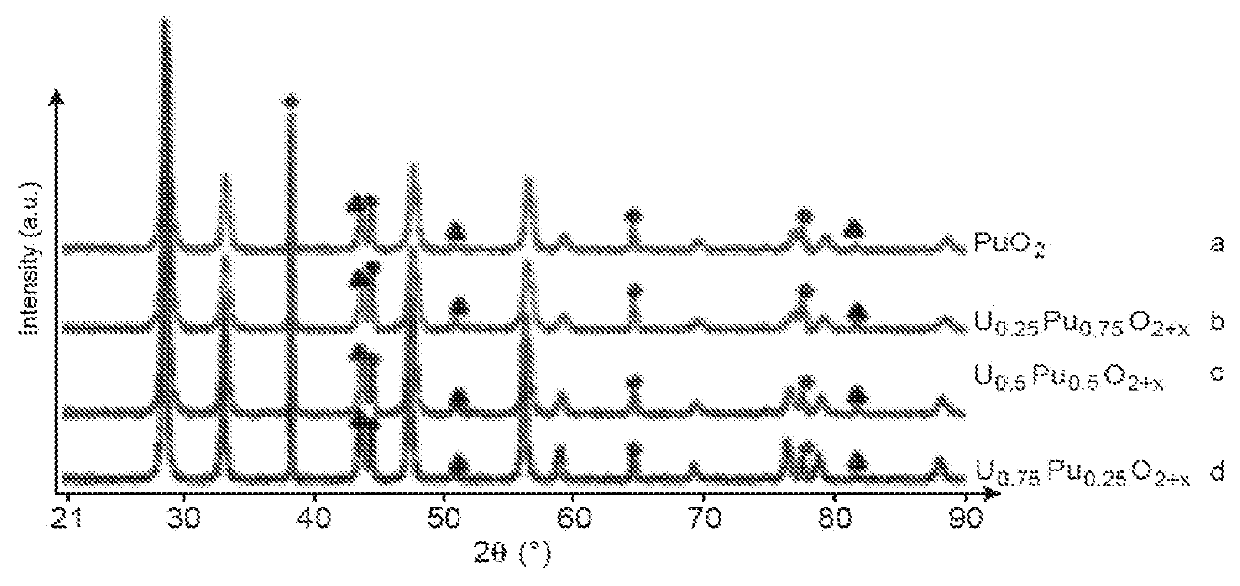
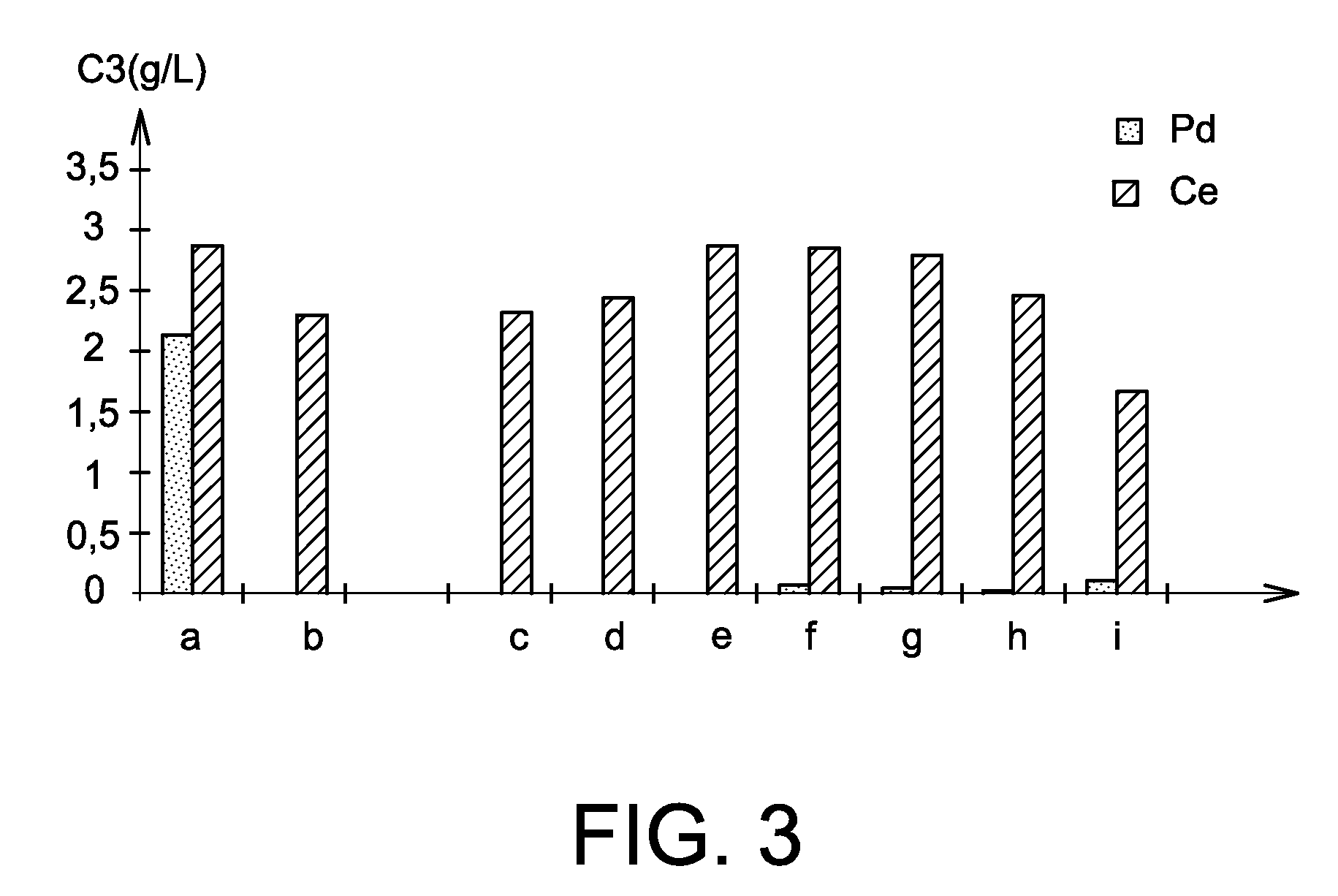
Process for separating at least one platinoid element from an acidic aqueous solution comprising, besides this platinoid element, one or more other chemical elements
The invention relates to a process for recovering at least one platinoid element contained in an acidic aqueous solution comprising chemical elements other than the platinoid element, the process comprising the steps of (a) bringing the acidic aqueous solution into contact with a reducing amount of a reducing agent which is a non-sulphurous and non-glucidic alcoholic compound chosen from cyclic, optionally aromatic, alcohols and aliphatic polyols, which reduces the platinoid element to its 0 oxidation state; and (b) separating the reduced platinoid element from the acidic aqueous solution.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com