Controlled synthesis method of uranium oxide microcrystal/nanocrystal
A synthesis method and nanocrystal technology, applied in the direction of uranium dioxide, uranium oxide/hydroxide, nanotechnology, etc., can solve the problems of lack of high quality, limitations of micro-nano materials, etc., and achieve uniform size, good crystallinity, The effect of good crystallinity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1a. Under slow electromagnetic stirring, dissolve uranyl acetate into a mixed solution of acetone and water, the volume percentage of acetone is 50%, to obtain solution I, and the concentration of uranyl ion in solution I is 1 mmol / L;
[0022] 1b. Add ammonia water to solution I, adjust the pH value to 9.5~11.0, and obtain solution II;
[0023] 1c. Transfer the solution II to a polytetrafluoroethylene reactor, react at 200°C for 72 hours, and naturally cool to room temperature to obtain a precipitate;
[0024] 1d. The precipitate is centrifuged and separated, and then washed with ethanol and acetone to obtain the product.
[0025] The phase of the product can be identified as pure UO2 by X-ray diffraction, and the crystallinity is >95%.
Embodiment 2
[0027] 2a. Under slow electromagnetic stirring, dissolve uranyl nitrate into a mixed solution of acetone and water, the volume percentage of acetone is 20%, to obtain solution I, and the concentration of uranyl ions in solution I is 10 mmol / L;
[0028] 2b. Adjust the pH value of solution I to 4.5 with ammonia water to obtain solution II;
[0029] 2c. Transfer the solution II to a polytetrafluoroethylene reactor, react at 160°C for 24 hours, and naturally cool to room temperature to obtain a yellow precipitate;
[0030] 2d. The precipitate is centrifuged and separated, and then washed with ethanol and acetone to obtain the product.
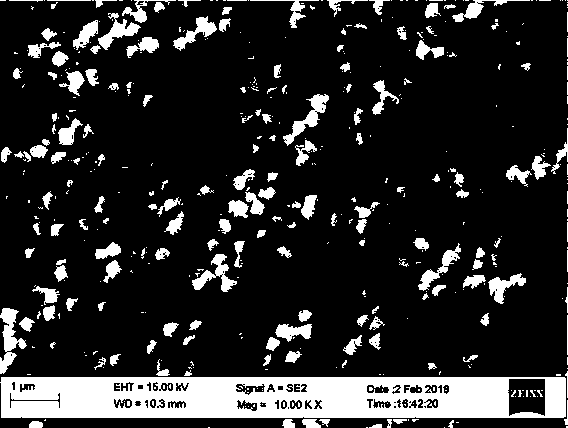
[0031] The phase of the product can be identified by X-ray diffraction as pure UO3·0.8H2O with a crystallinity of >95%. The shape of hexagonal microflakes was observed by scanning electron microscopy.
Embodiment 3
[0033] 3a. Under slow electromagnetic stirring, dissolve uranyl acetate into a mixed solution of acetone and water, the volume percentage of acetone is 50%, to obtain solution I, and the concentration of uranyl ion in solution I is 10mmol / L;
[0034] 3b. Add ammonia water to solution I, adjust the pH value to 7.5~8.5, and obtain solution II;
[0035] 3c. Transfer the solution II to a polytetrafluoroethylene reactor, react at 200°C for 20 hours, and naturally cool to room temperature to obtain a precipitate;
[0036] 3d. The precipitate is centrifuged and separated, and then washed with ethanol and acetone to obtain a product.
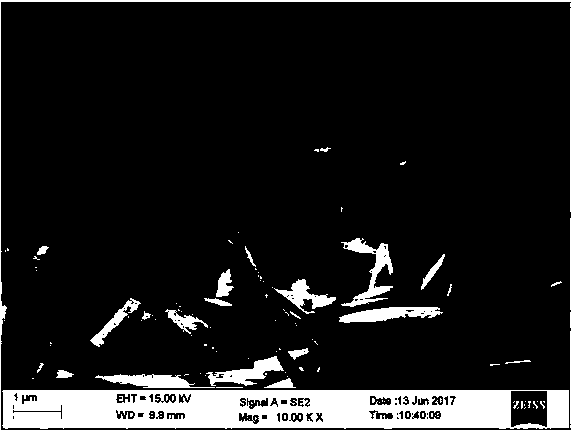
[0037] The phase of the product can be identified by X-ray diffraction as pure U3O8 with crystallinity>95%. Scanning electron microscope observed such as figure 1 The shape shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com