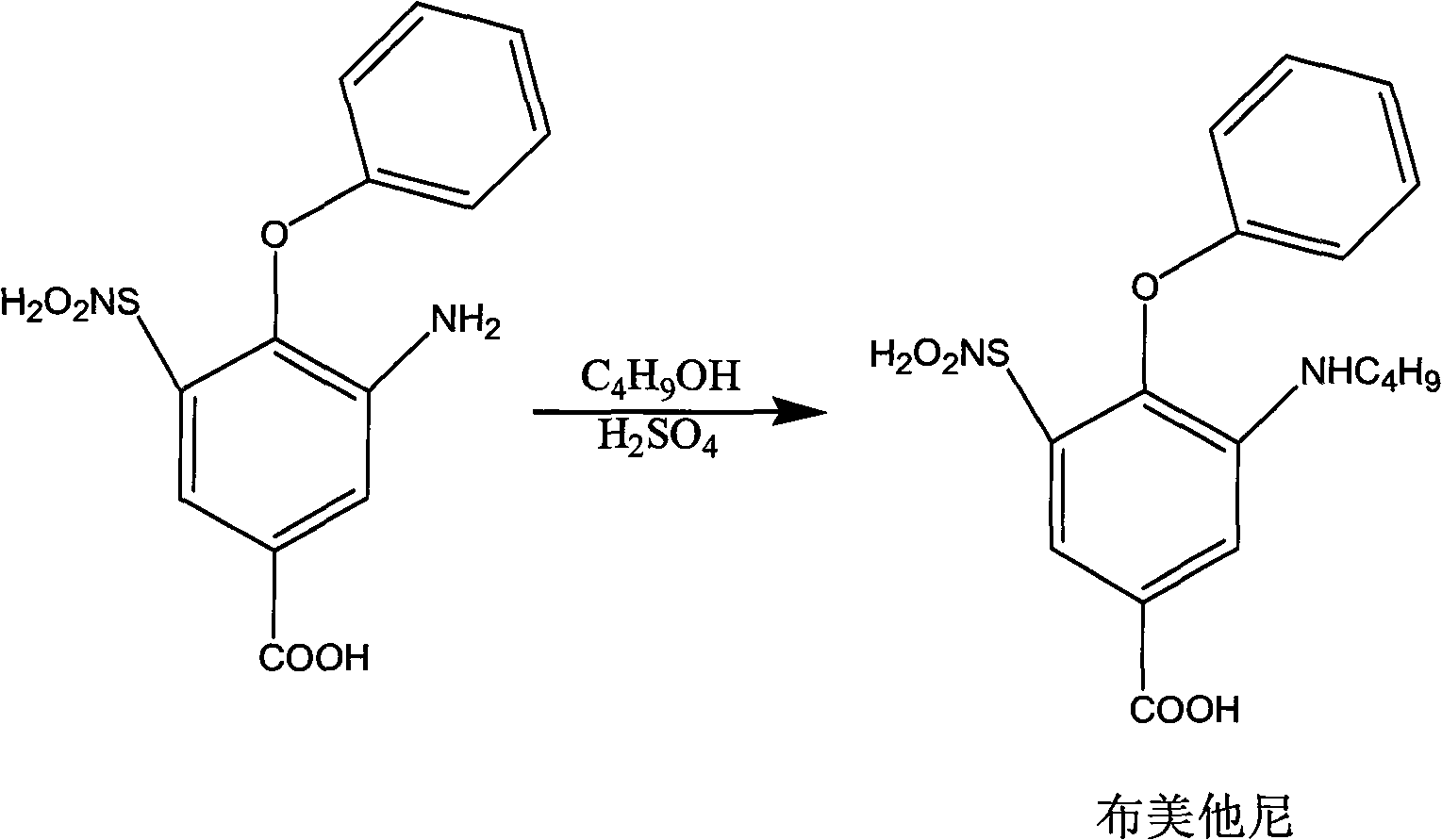
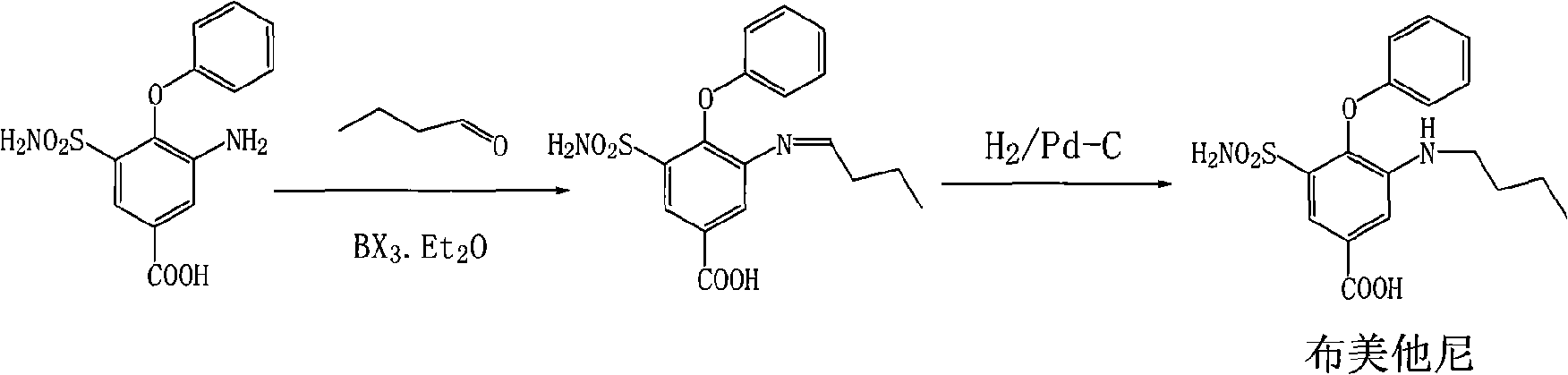
Method for preparing bumetanide
A technology of phenoxy and sulfonamido benzoic acid is applied in the preparation of sulfonic acid amides, medical preparations containing active ingredients, pharmaceutical formulations, etc., and can solve the problems of long reaction time, cumbersome post-processing, unsuitable for industrialized production and the like, Achieve the effect of low equipment requirements and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Combine 60g 3-amino-4-tolyloxy-5-sulfonamidobenzoic acid, 22ml n-butyraldehyde, 42ml boron trifluoride ether (containing about 47% boron trifluoride), 600ml methanol and 10g 5% palladium on carbon Add to a 1000ml autoclave and replace it with nitrogen and hydrogen three times respectively, so that the materials in the kettle will react at a temperature of 20℃~25℃ and a hydrogenation pressure of 1~2atm. After about 14h, the pressure in the reactor will not change. Indicates the end of hydrogen absorption. Discharge, filter and recover palladium-carbon. After adding 6g activated carbon to decolorize the filtrate for 10 minutes, filter, keep the filtrate, add the filtrate dropwise to 2400ml of water, precipitate solids, filter, wash the filter cake with water, and drain to obtain 66g of white solids. Bumetanide. The yield was 93%.
Embodiment 2
[0024] Add 60g 3-amino-4-tolyloxy-5-sulfonamidobenzoic acid, 22ml n-butyraldehyde, 42ml boron trichloride ether, 600ml ethanol and 10g 5% palladium charcoal into a 1000ml autoclave and use Replace with nitrogen and hydrogen three times, at a temperature of 20°C to 25°C and a pressure of 1 to 2 atm until the end of hydrogen absorption, about 14 hours. After the reaction is complete, the material is discharged and the palladium-carbon is recovered by filtration. The filtrate is added with 6g of activated carbon and refluxed for 10 minutes, and then filtered. The obtained filtrate is added dropwise to 2400ml of water to precipitate solids, filtered, the filter cake is washed with water and drained to obtain 64g of white solids, which is cloth Metanide, the yield is 90%.
Embodiment 3
[0026] Add 60g 3-amino-4-tolyloxy-5-sulfonamidobenzoic acid, 22ml n-butyraldehyde, 42ml boron trifluoride ether, 600ml methanol and 10g 10% palladium charcoal into a 1000ml autoclave and use Replace with nitrogen and hydrogen three times, at a temperature of 20°C to 25°C and a pressure of 1 to 2 atm until the end of hydrogen absorption, about 6 hours. After the reaction is completed, the reactants are filtered to recover the palladium-carbon. The reaction solution is added with 6g activated carbon and refluxed for 10 minutes, and then filtered. The resulting filtrate is added dropwise to 2400ml of water to separate out solids. Filter, wash the filter cake with water and drain to obtain 68g of white solids It is bumetanide with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com