Preparation method of phosphous dihalide with substituent group
A technology of phosphorus dihalide and phosphoryl phosphorus trihalide, which is applied in chemical instruments and methods, organic chemistry, and compounds of Group 5/15 elements of the periodic table, etc., which can solve the difficulties of product separation and purification, unstable process routes, and equipment Harsh requirements and other issues, to achieve the effect of high reaction yield, easy industrial implementation, and no three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
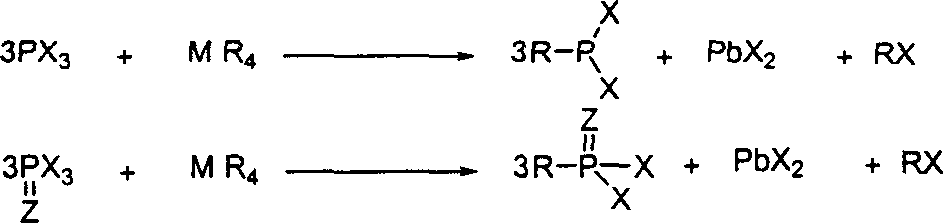
[0028] Add 412 grams of phosphorus trichloride and 515 grams of tetraphenyl lead successively in a nitrogen-dried reaction flask equipped with mechanical stirring, a constant pressure dropping funnel, and a thermometer. Vigorously stirred at 180°C, a gray precipitate was produced as the reaction progressed, and the reaction was stopped after stirring for 180 hours. Directly carry out vacuum distillation to obtain 500 grams of phenylphosphorous dichloride, the yield is 93%, 31 P NMR (CDCl 3 , 100MHz): -161.8ppm
Embodiment 2
[0030] Add 731 grams of phosphorus tribromide successively in a nitrogen-dried reaction flask equipped with mechanical stirring, a constant pressure dropping funnel, and a thermometer, and then add 323 grams of tetraethyl lead dropwise to the reaction system. During the dropwise addition, Accompanied by a violent exotherm. Vigorously stirred at 20°C, a gray precipitate was produced as the reaction progressed, and the reaction was stopped after stirring for 35 hours. Directly carry out vacuum distillation to obtain 356 grams of ethyl phosphorus dibromide, and the yield is 60%, 31 P NMR (CDCl 3 , 100MHz): -194.8ppm.
Embodiment 3
[0032] Add 458 grams of sulfuryl phosphorus trichloride successively in a nitrogen-dried reaction flask equipped with mechanical stirring, a constant pressure dropping funnel, and a thermometer, and then slowly add 323 grams of tetraethyl lead dropwise to the reaction system. After the dropwise addition, stir vigorously at 20°C for one hour, then gradually raise the temperature to 70°C, and continue to stir for 45 hours. As the reaction proceeds, gray precipitates are formed. After continuing to stir for 19 hours, stop the reaction. Directly carry out vacuum distillation to obtain 402 grams of sulfuryl ethyl phosphorus dichloride. (Yield: 90%) 31 P NMR (CDCl 3 , 100MHz): -94.3ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com