Flexible nanoliposomes with charges for cosmetics and preparation method thereof
A nano-liposome, charged technology, applied in cosmetic preparations, cosmetics, cosmetic preparations and other directions, can solve the problems of not easy to promote transdermal absorption, low self-stability, etc., and achieve good skin permeability, The effect of high encapsulation efficiency, drug loading, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
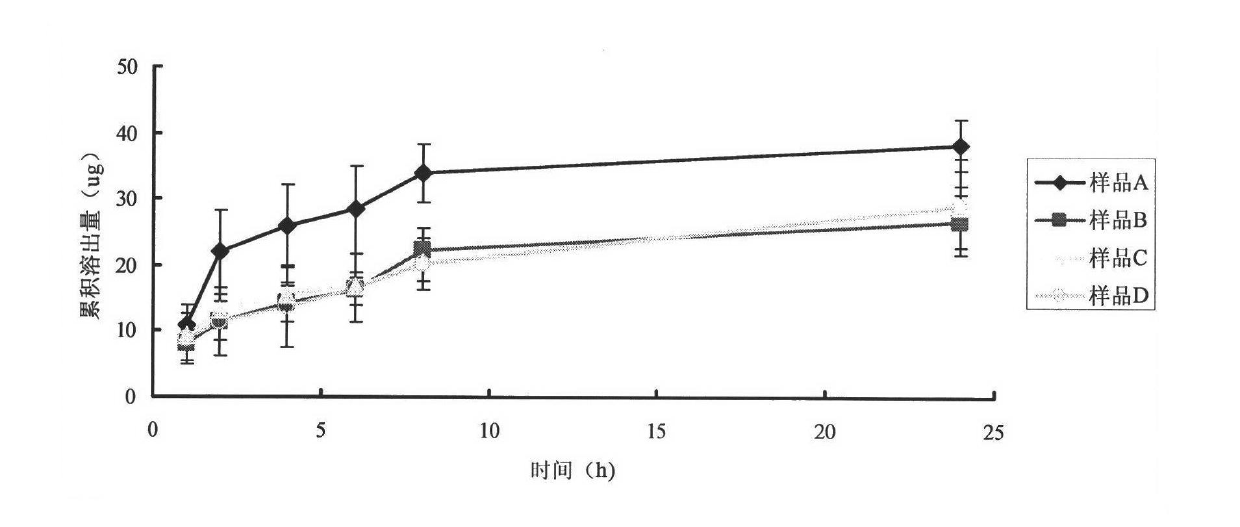
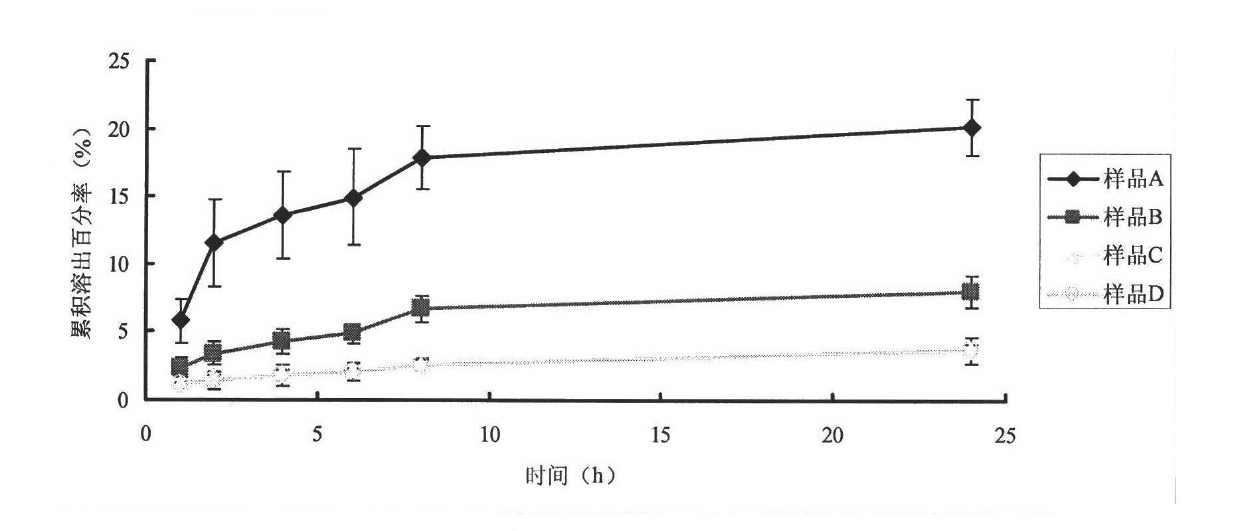
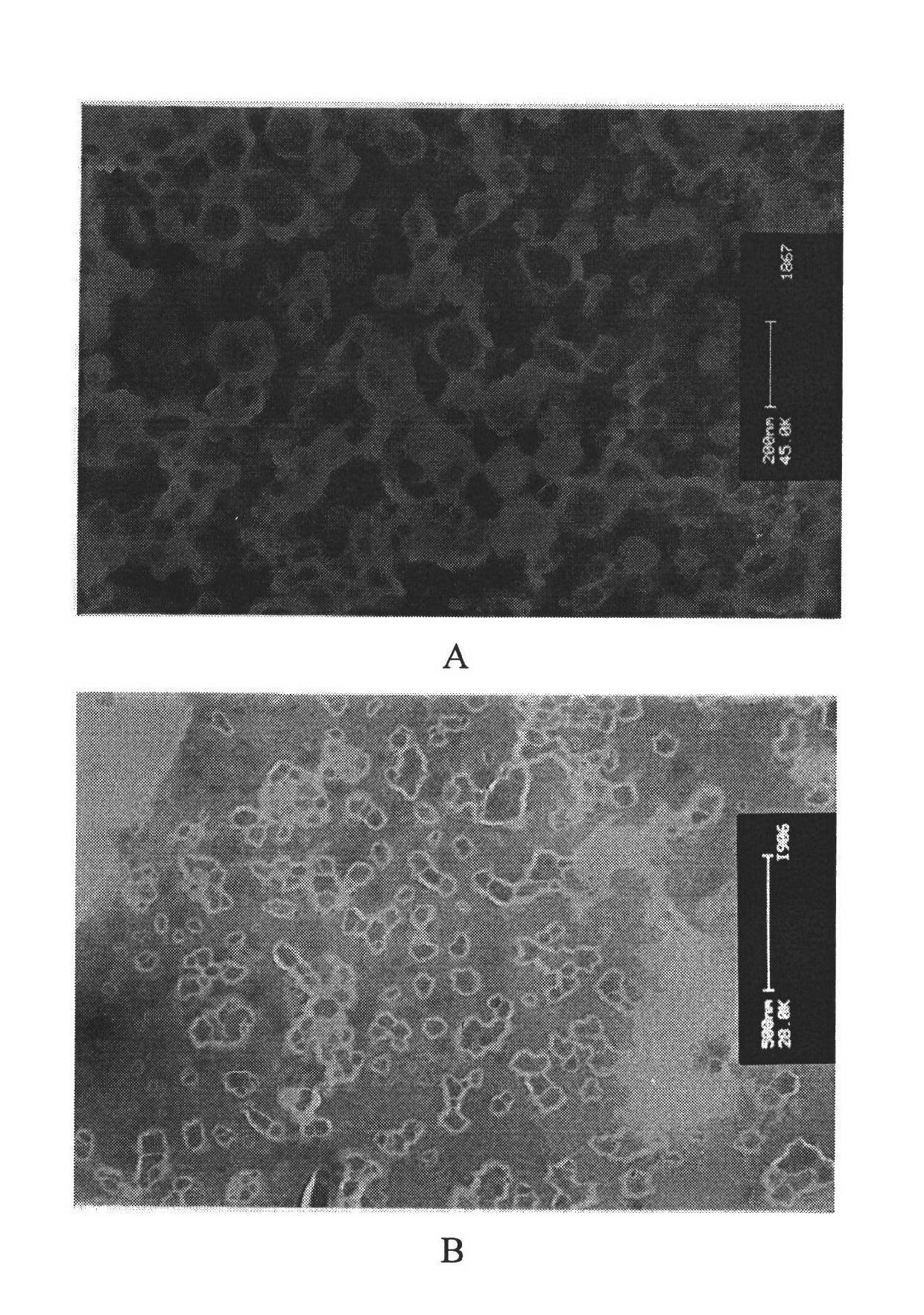
[0037] Dissolve 100mg of soybean lecithin, 16mg of stearylamide, and 15mg of cholesterol in chloroform by ultrasonication in a water bath, dissolve 25mg of asiaticoside, 0.26mg of vitamin E and BHA mixed antioxidants in a small amount of ethanol, and mix with chloroform; Sodium cholate and 1.7 mg hyaluronic acid were dissolved in phosphate buffer at pH 7.0; the aforementioned phosphate buffer and chloroform solution were sonicated in a water bath to form a uniform emulsion, and after standing for 30 minutes, rotary evaporation obtained lipid body suspension; the liposome suspension was ultrasonicated for 30 cycles with a probe-type ultrasonic cell disruptor (5 seconds per cycle, 5 seconds at rest), homogenized 3 times with an AVESTIN high-pressure homogenizer, and passed through The AVESTIN extruder passes through a 100nm microporous membrane 10 times, and the liposome suspension extruded is put into a dialysis bag (molecular cut-off 8000-14000) for dialysis for 6 hours; the li...
Embodiment 2
[0040] 120mg of hydrogenated soybean lecithin, 20mg of stearylamide, 18mg of cholesterol were ultrasonically dissolved in chloroform, and 0.25mg of vitamin E and BHA mixed antioxidants were dissolved in a small amount of ethanol, and then mixed with chloroform; 2000UI epidermal active factor ( EGF), 15 mg sodium cholate, and 1.7 mg hyaluronic acid were dissolved in phosphate buffer at pH 7.0; the aforementioned phosphate buffer and chloroform solution were ultrasonically formed into a uniform emulsion in a water bath, and after standing for 30 minutes, rotate Evaporate to get liposome suspension; After liposome suspension is ultrasonically 30 cycles (every cycle ultrasonic 5 seconds, intermittent 5 seconds) with probe type ultrasonic cell disruptor, use AVESTIN high-pressure homogenizer homogeneous 3 time, and then through the microporous membrane of 100nm by AVESTIN extruder 10 times, the liposome suspension extruded was put into dialysis bag (molecular cut-off 8000-14000) and...
Embodiment 3
[0043] Dissolve 10mg of dipalmitoylphosphatidylcholine, 2mg of stearamide, and 2mg of cholesterol in chloroform by ultrasonication in a water bath, dissolve 0.20mg of vitamin C and BHA mixed antioxidants in a small amount of ethanol, and mix with chloroform; Grass root, 1.6 mg sodium cholate, and 1.7 mg hyaluronic acid were dissolved in phosphate buffer solution with pH 7.0; the aforementioned phosphate buffer solution and chloroform solution were ultrasonically formed into a uniform emulsion in a water bath, and after standing for 30 minutes, The liposome suspension was obtained by rotary evaporation; the liposome suspension was ultrasonicated for 30 cycles with a probe-type ultrasonic cell disruptor (5 seconds per cycle, 5 seconds at rest), and then homogenized with an AVESTIN high-pressure homogenizer 3 times, then through the microporous filter membrane of 100nm by AVESTIN extruder 10 times, the liposome suspension of extruding is put into dialysis bag (molecular cut-off 80...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com