Method for preparing ropinirole
A compound and reaction technology, applied in the field of preparation of ropinirole hydrochloride, can solve the problems of weak positioning, unsuitable for industrial production, difficult to purify and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
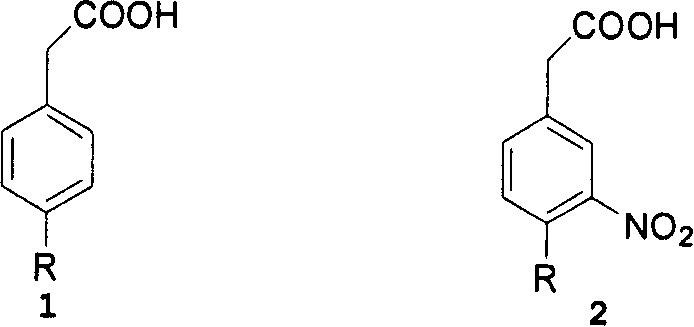
[0053] Example 1 3-nitro-4-chlorophenylacetic acid
[0054] Completely dissolve 68.2 g of 4-chlorobenzoic acid into 800 mL of concentrated sulfuric acid, add 20 mL of fuming nitric acid at 0°C, and then stir for 1 hour. After the reaction was completed, the reaction solution was slowly poured into 1000 mL of ice water, stirred for 30 minutes, and then filtered. The filter cake was stirred with 2000 mL of ice water in the reaction kettle for 30 minutes, then filtered, washed with ice water until neutral, and then vacuum-dried at 50°C to obtain 75.14 g of the title compound as a light yellow solid, with a yield of 88.7%. 1 HNMR (300MHz, DMSO-d6): 7.82 (d, J = 2.7, 1H), 7.52 (d, J = 8.1, 1H), 7.44 (dd, J = 8.1, 2.7, 1H), 3.72 (s, 2H) .
Embodiment 2
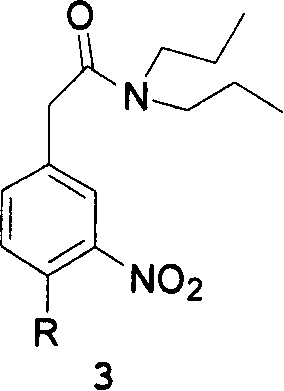
[0055] Example 2 N, N-dipropyl-3-nitro-4-chlorophenylacetamide
[0056] 21.3 grams of 3-nitro-4-chlorophenylacetic acid and 200 mL of SOCl2 were added into a 500 mL reaction flask, and the reaction was refluxed for 2.5 hours. After the reaction, SOCl2 was recovered to obtain light brown oil.
[0057]Add 100 mL of ethyl acetate to the oily substance, dissolve it and drop it into the ethyl acetate solution containing 27.3 mL of dipropylamine at room temperature, white smoke will be produced when the dropwise addition starts. After the addition was complete, stir at room temperature for 3 hours. Then add 100 mL of 5% NaOH solution to wash, then wash with 100 mL of 10% hydrochloric acid, and separate the organic layer. The solvent was recovered to obtain the title compound as a light brown oil, with a yield of 84.8% and a purity of 96.4%. The oil was used directly in the next step.
Embodiment 3
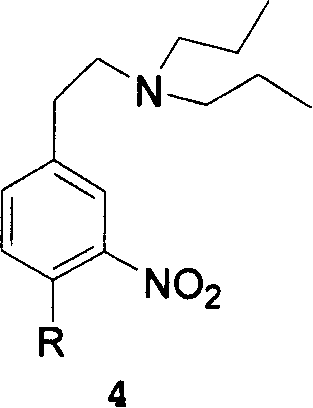
[0058] Example 3 N, N-dipropyl-3-nitro-4-chlorophenethylamine
[0059] Dissolve 15 g of N,N-dipropyl-3-nitro-4-chlorophenylacetamide in 100 mL of THF, and then add 5 g of LiAlH4 in batches at room temperature. React overnight at room temperature. 15mL of ice water was added dropwise, and a large amount of gas was released at the beginning. After the addition, the solution became a paste and was filtered. The organic phase was dried over anhydrous MgSO4 and the solvent was evaporated to give 14.3 g of the title compound in an almost quantitative yield with a purity of 96.1%. 1 HNMR (400MHz, DMSO-d6): 7.93 (1H, d, J = 2.4Hz), 7.68 (1H, d, J = 8.4Hz), 7.54 (1H, dd, J 1 =8.4Hz,J 2 =2.4Hz), 3.82(2H, s), 3.26(2H, m), 3.18(2H, m), 1.52(2H, m), 1.44(2H, m), 0.854(3H, t, J=7.3Hz ), 0.776 (3H, t, J = 7.6 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com