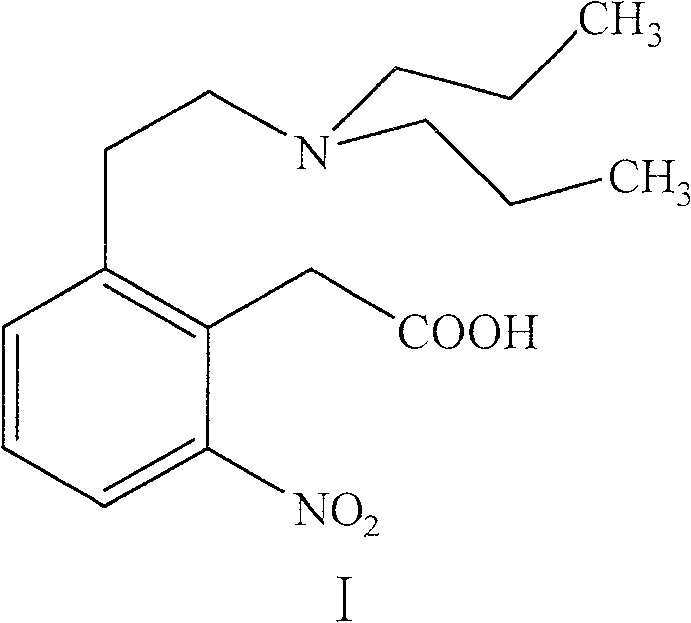
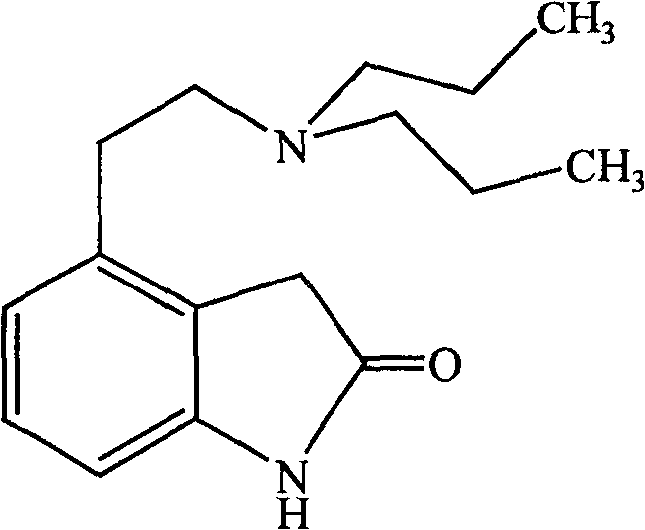
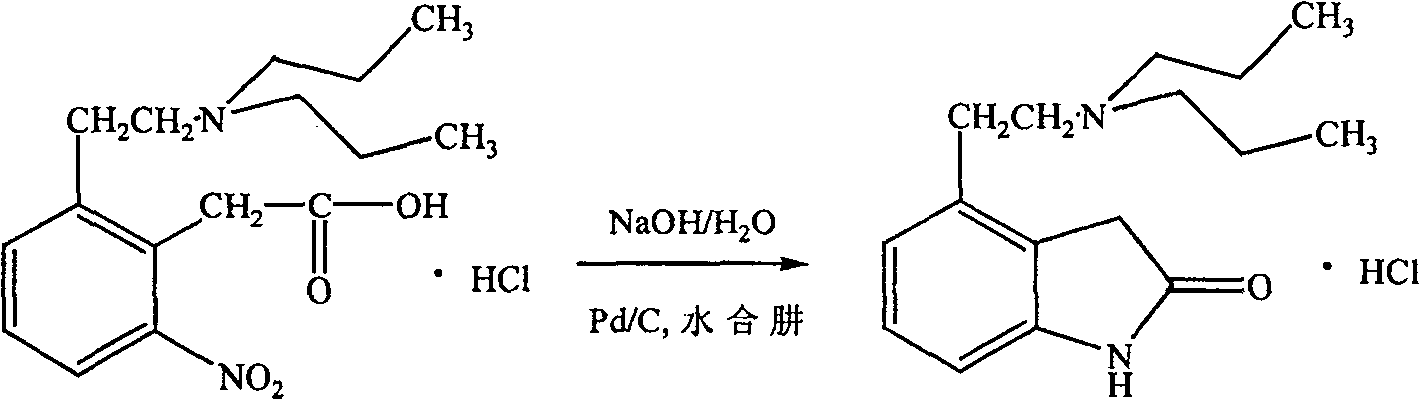
Process for preparing ropinirole and its salt
A technology for ropinirole hydrochloride and a compound is applied in the field of preparation of ropinirole and its salts, and achieves the effects of strong controllability, mild reaction conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation of embodiment 1 ropinirole hydrochloride
[0018] 38 grams of intermediate I, 100 milliliters of water, 300 milliliters of 95% ethanol and 280 grams of zinc powder were mixed and stirred. Raise the temperature to reflux for 4 hours, then heat filter to remove unreacted zinc sludge and zinc oxide produced by the reaction. 20 ml of concentrated hydrochloric acid was added to the filtrate to reflux for 2 hours, most of the ethanol was evaporated from the reaction solution under reduced pressure, and the residue was decolorized with activated carbon. Filter, cool the filtrate, and adjust the pH value to 8-9 with sodium hydroxide solution. Dichloromethane extraction, 200 milliliters of isopropanol and 20 milliliters of concentrated hydrochloric acid were added to the extract, most of the solvent was evaporated under reduced pressure, the residue was cooled at room temperature, and a yellow solid was precipitated, which was filtered to obtain 18 grams of a li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com