Implantable devices for drug delivery with reduced burst release
An implanted device and delivery technology, applied in drug delivery, drug devices, medical preparations containing active ingredients, etc., can solve problems such as prohibition, inconvenient compliance with drug regimens, and intolerable intestinal drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] Preparation of device of the present invention
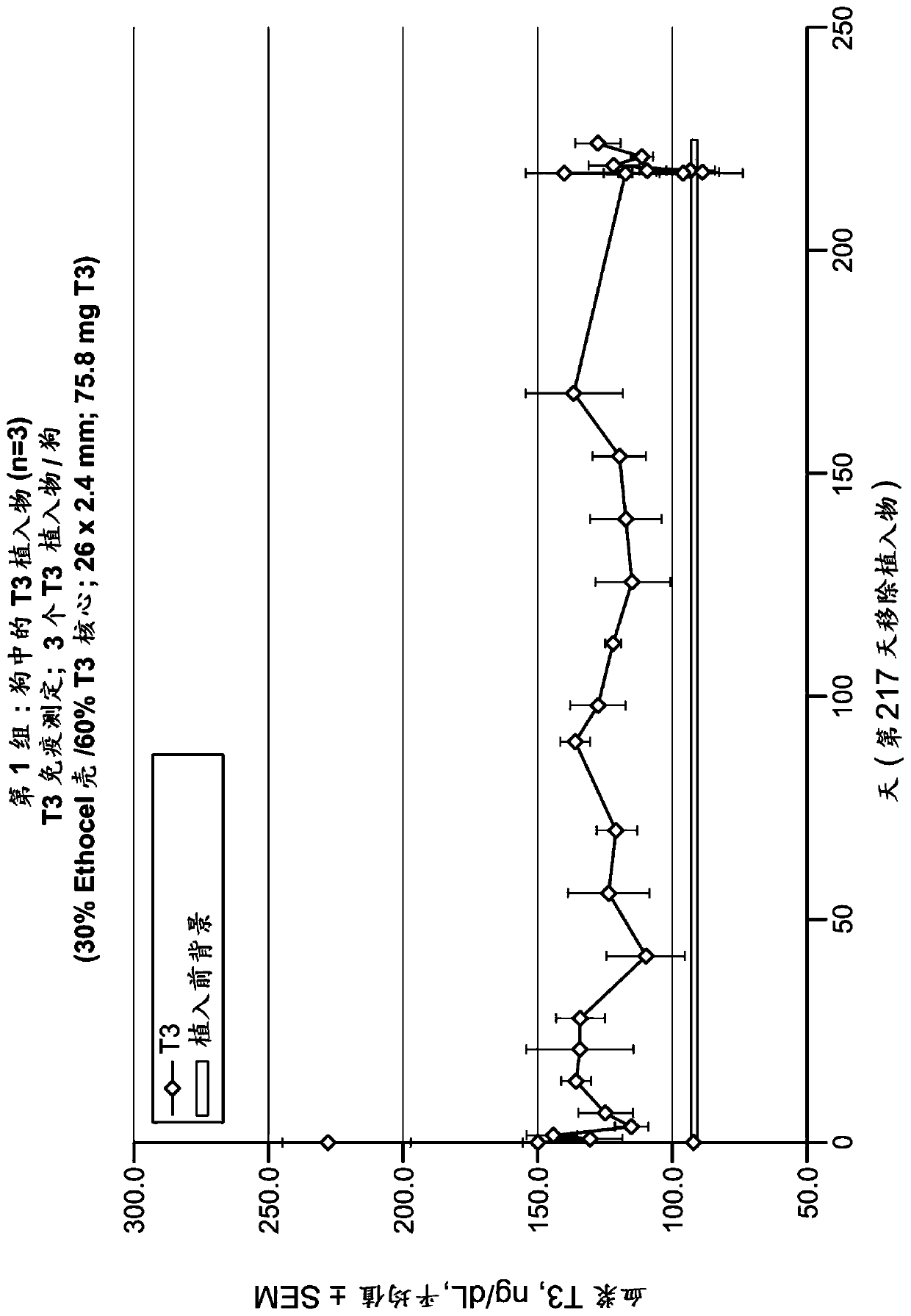
[0142] In some embodiments, implantable devices of the present invention can be prepared by coextruding the drug-containing core and porogen-containing shell of the device. The pharmaceutical substance is reduced to fine particles by milling (eg, ball milling, impact milling), spray drying, solvent precipitation, sieving, or other method or combination of methods known in the art to produce fine particles. The drug can be combined with a polymer that is also made into fine particles to form the mixture used to make the drug-containing core. Likewise, porogen-containing shells are prepared by blending fine particles of polymer with porogen particles of the desired size. Each blended mixture is heated to a temperature suitable for extrusion, such as the softening point of the polymer. At this point, optionally and if necessary, either or both of the softened mixtures can be homogenized. The mixture is then coextruded, fo...
Embodiment approach 1
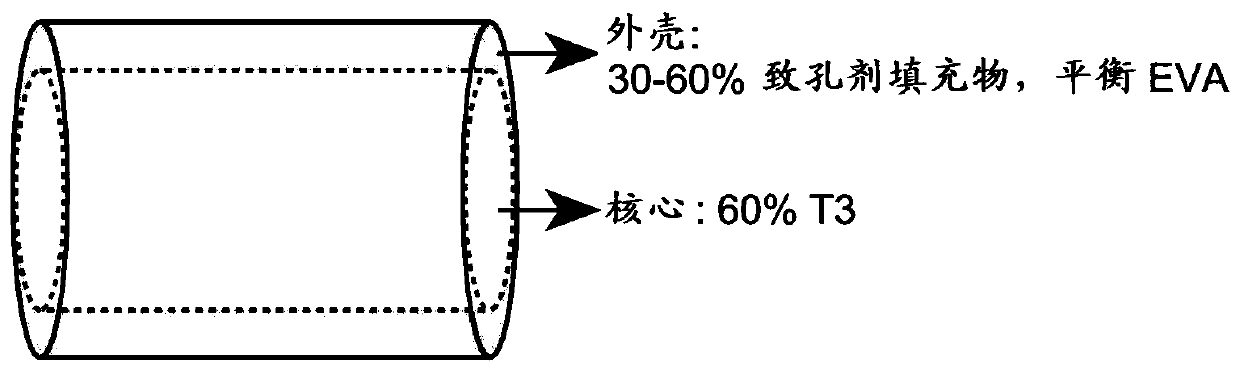
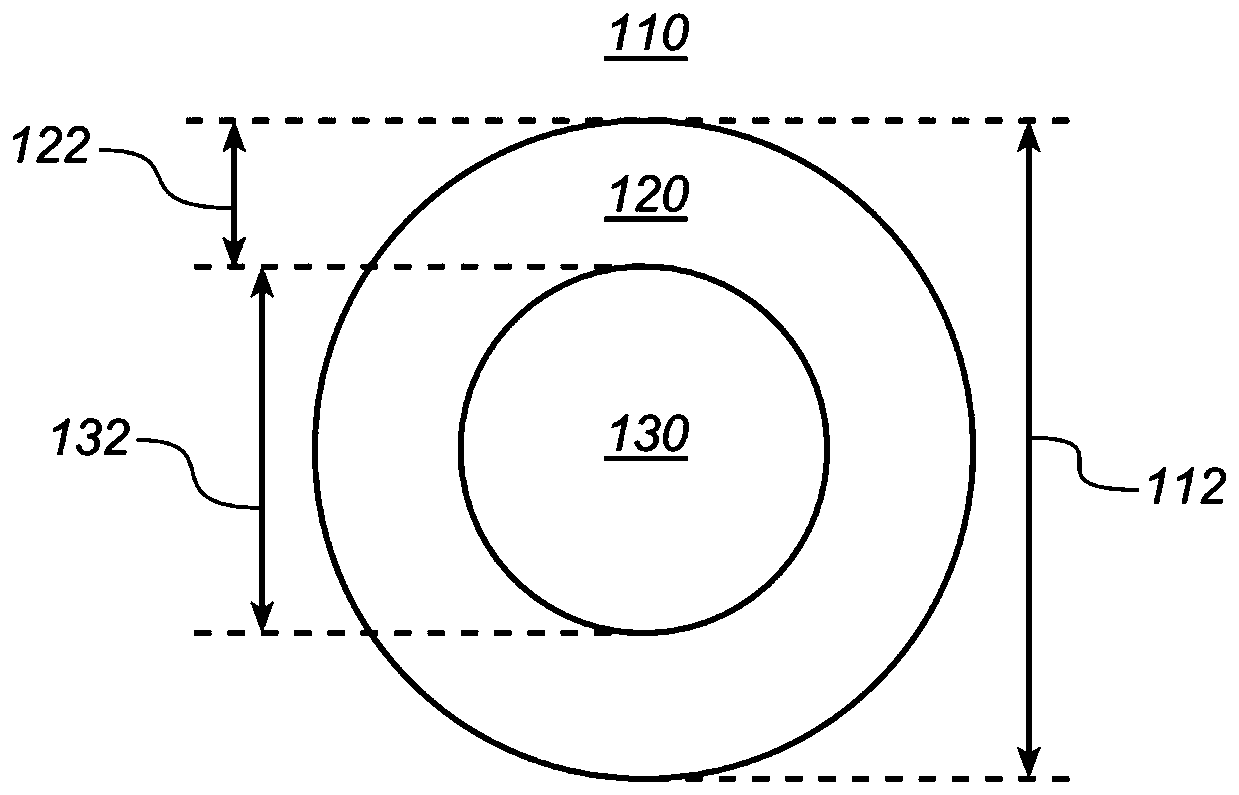
[0161] Embodiment 1. An implantable device for delivering a drug substance comprising a core comprising a first polymer material and a core drug substance; and a shell comprising a second polymer material and a porogen material; wherein, with The implantable device has a reduced burst release compared to a comparative device made entirely of the first polymer material and the core drug substance.
Embodiment approach 2
[0162] Embodiment 2. The implantable device of embodiment 1, wherein the shell is a drug-free layer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com