Osmotic pumping sustained preparation of ropinirole hydrochloride and its preparing method
A technology of controlled-release preparations and osmotic pumps, applied in the field of ropinirole hydrochloride osmotic pump-type controlled-release preparations and its preparation, can solve problems such as static tremor, difficulty walking, and inability to fit, and achieve stable blood drug concentration and eliminate Peak and valley phenomenon, the effect of reducing symptom fluctuation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
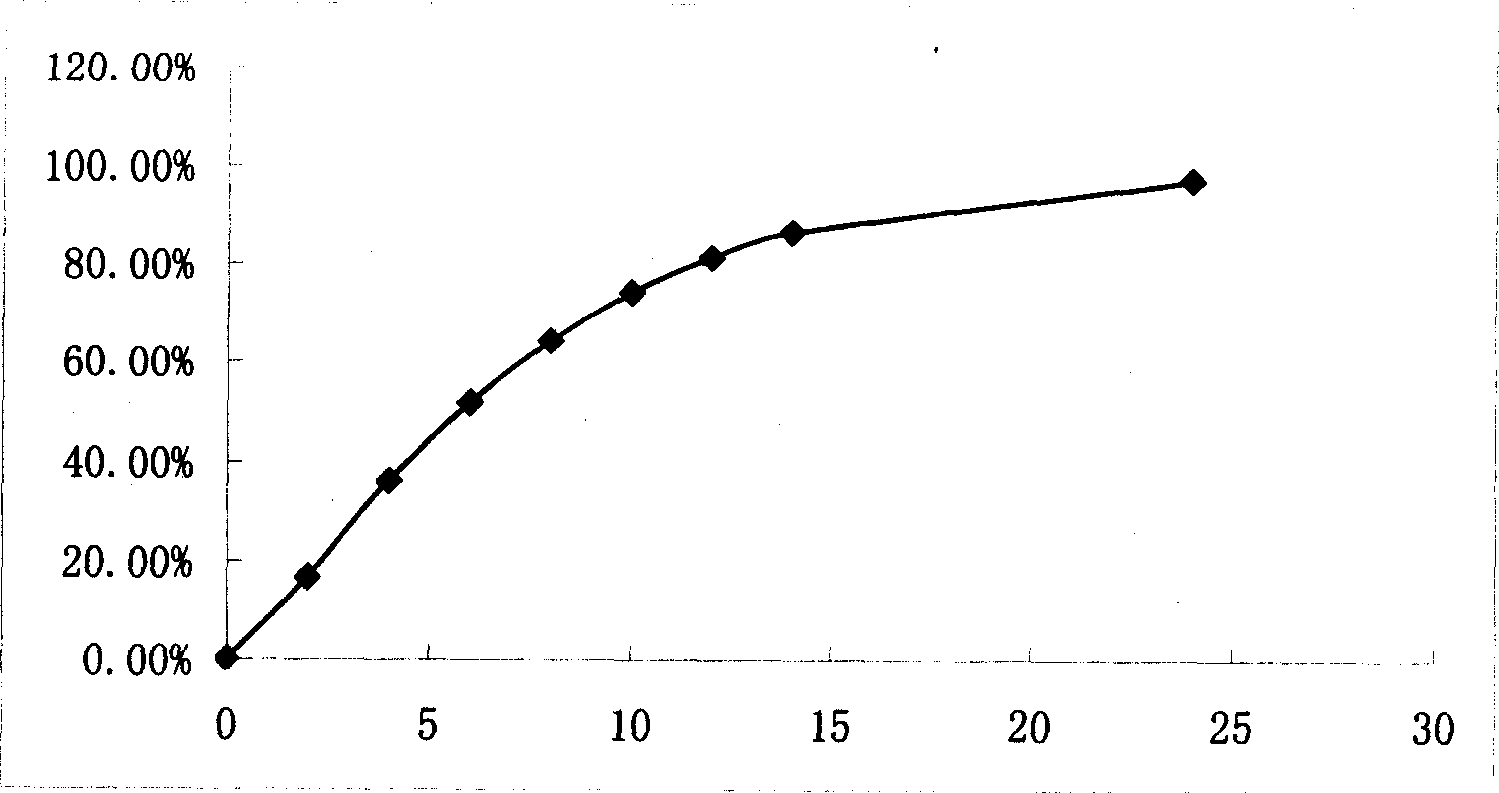
Examples
Embodiment 1
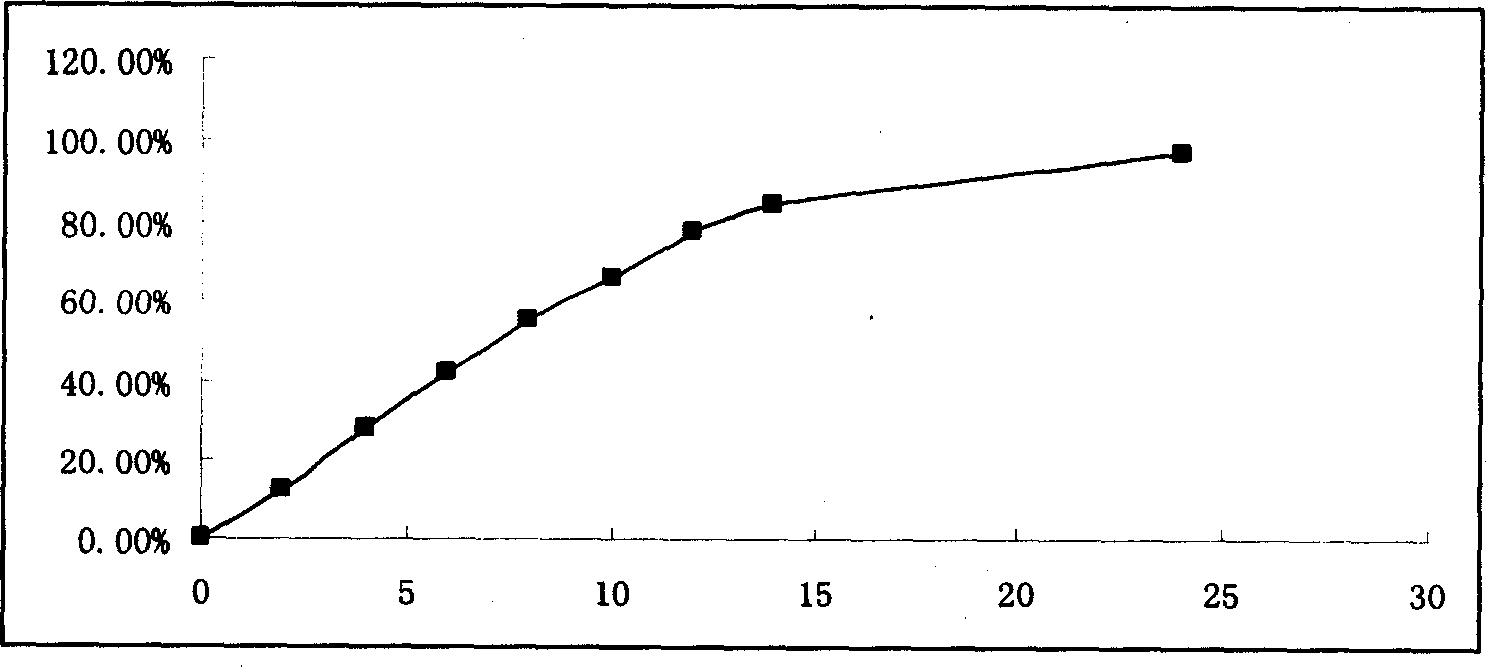
[0021] Example 1 Osmotic pump controlled release tablet using lactose and mannitol in combination as osmotic pressure enhancer
[0022] Tablet prescription:
composition
Prescription A (mg / tablet)
Prescription B (mg / tablet)
13.68
27.36
89
178
89
178
pvp
4
8
Cross-linked PVP
4
8
1.6
3.2
Theoretical core weight
201
402
Coating prescription:
Cellulose acetate
10g
10g
Polyethylene glycol-1500
0.8108g
0.8108g
[0023] Preparation: sieve and mix the prescribed amount of medicine with lactose, mannitol, PVP, and glue-linked PVP, use 90% ethanol to make a soft material, granulate with a 20-mesh sieve, dry in an oven at 40°C for 12 hours, and sieve through a 20-mesh sieve Granules, dry granules plus ...
Embodiment 2
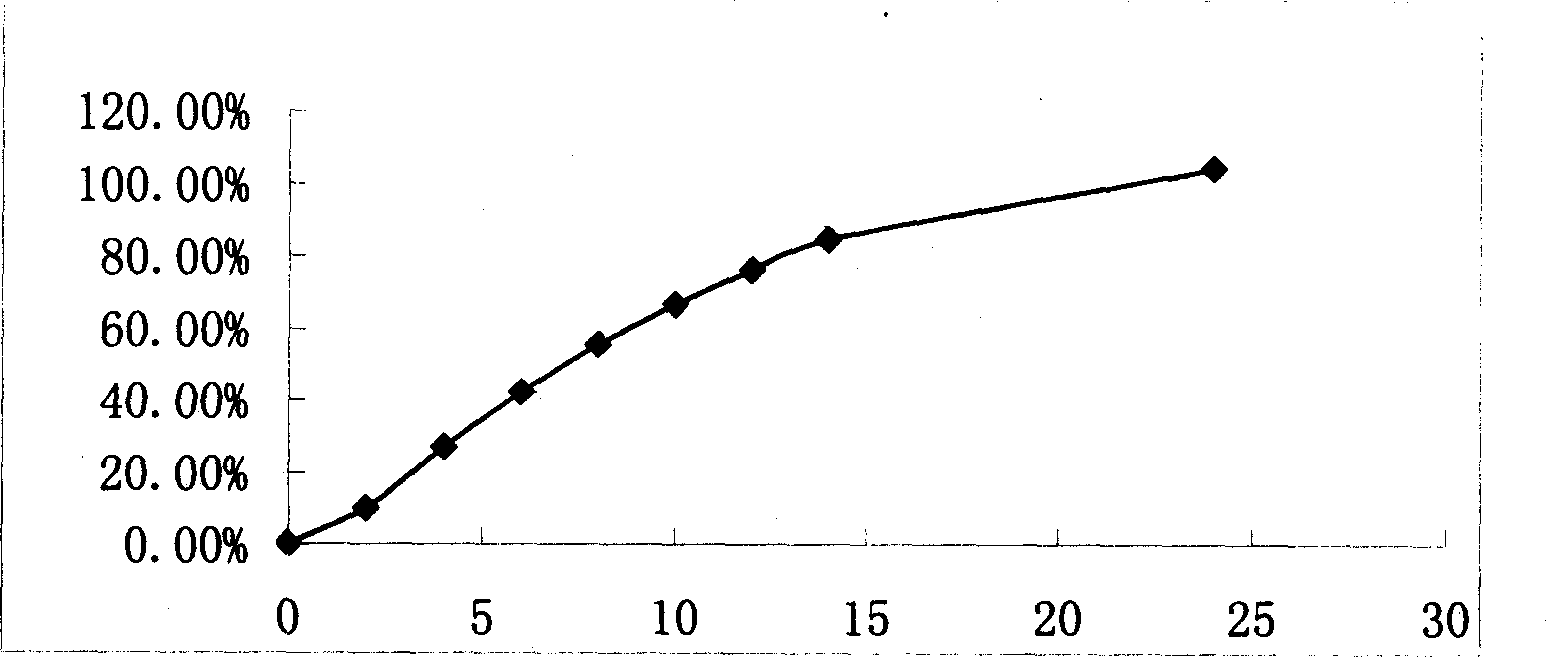
[0025] Example 2 Osmotic pump controlled release tablet using lactose and mannitol in combination as osmotic pressure enhancer
[0026] Tablet Prescription:
Prescription A (mg / tablet)
Prescription B (mg / tablet)
6.84
3.42
lactose
92
93
92
93
pvp
4
4
Glue-linked PVP
4
4
1.6
1.6
Theoretical film weight
209.5
209.2
Coating prescription:
Cellulose acetate
10g
Polyethylene glycol-1500
0.8108g
[0027] Preparation: sieve and mix the prescribed amount of medicine with lactose, mannitol, PVP, and glue-linked PVP, use 90% ethanol to make a soft material, granulate with a 20-mesh sieve, dry in an oven at 40°C for 12 hours, and sieve through a 20-mesh sieve Granules, dry granules plus lubricant magnesium stearate compressed into tablet c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com