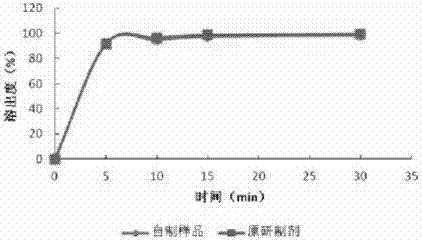
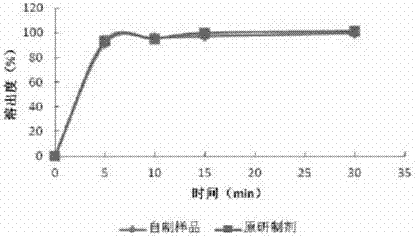
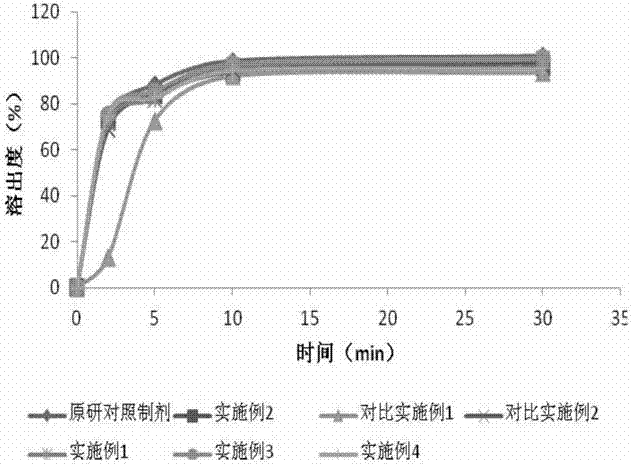
Preparation method of composition containing ropinirole
A compound and mixture technology, applied in the field of preparation of pharmaceutical compositions, can solve the problems of complex preparation process and poor content uniformity of ropinirole preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: Preparation of ropinirole hydrochloride tablets by ropinirole hydrochloride-microcrystalline cellulose mixing-powder direct compression process
[0071] Take an appropriate amount of ropinirole hydrochloride, add 90% ethanol solution to dissolve, pour the solution into an appropriate amount of microcrystalline cellulose, stir and mix, and dry at 40°C for 4 hours; the dried powder is cross-linked with carboxymethyl cellulose with other auxiliary materials Sodium sodium, lactose and magnesium stearate were mixed, and the proportions of ropinirole hydrochloride, microcrystalline cellulose, croscarmellose sodium, lactose and magnesium stearate in the prescription were kept at 0.38%, 46.56%, 46.56%, 6% and 0.5%, compressed into tablets, and then coated with Opadry II 85G coating material, controlling the coating weight gain between 2% and 3%.
Embodiment 2
[0072] Embodiment 2: Preparation of ropinirole hydrochloride tablets by ropinirole hydrochloride-microcrystalline cellulose mixing-powder direct compression process
[0073] Take an appropriate amount of ropinirole hydrochloride, add 75% ethanol solution to dissolve, pour the solution into an appropriate amount of microcrystalline cellulose, stir and mix, and after mixing, place it in a hot air circulation drying oven for 2 hours at room temperature; the dried powder and prescription Other excipients in croscarmellose sodium, lactose and magnesium stearate are mixed to keep ropinirole hydrochloride, microcrystalline cellulose, croscarmellose sodium, lactose and magnesium stearate in the prescription The proportions in the prescription are 0.76%, 61.16%, 30.58%, 7% and 0.5% respectively, compressed into tablets, and then coated with Opadry II 85G coating material, controlling the weight gain of the coating at 2%-3% between.
Embodiment 3
[0074] Embodiment 3: Preparation of ropinirole hydrochloride tablets by ropinirole hydrochloride-silicon dioxide mixing-powder direct compression process
[0075] Take an appropriate amount of ropinirole hydrochloride, add an appropriate amount of water to dissolve, pour the solution into an appropriate amount of silicon dioxide, stir and mix, and dry at 40°C after mixing; the dried powder is cross-linked with other excipients in the prescription. Sodium cellulose, lactose and magnesium stearate are mixed, except that the microcrystalline cellulose in the embodiment 1 is replaced by silicon dioxide, keep the composition of the prescription and the composition of the prescription in the embodiment 2 the same, compress tablets, then use European Coating with Badai II 85G coating material, control the weight gain of the coating between 2% and 3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com