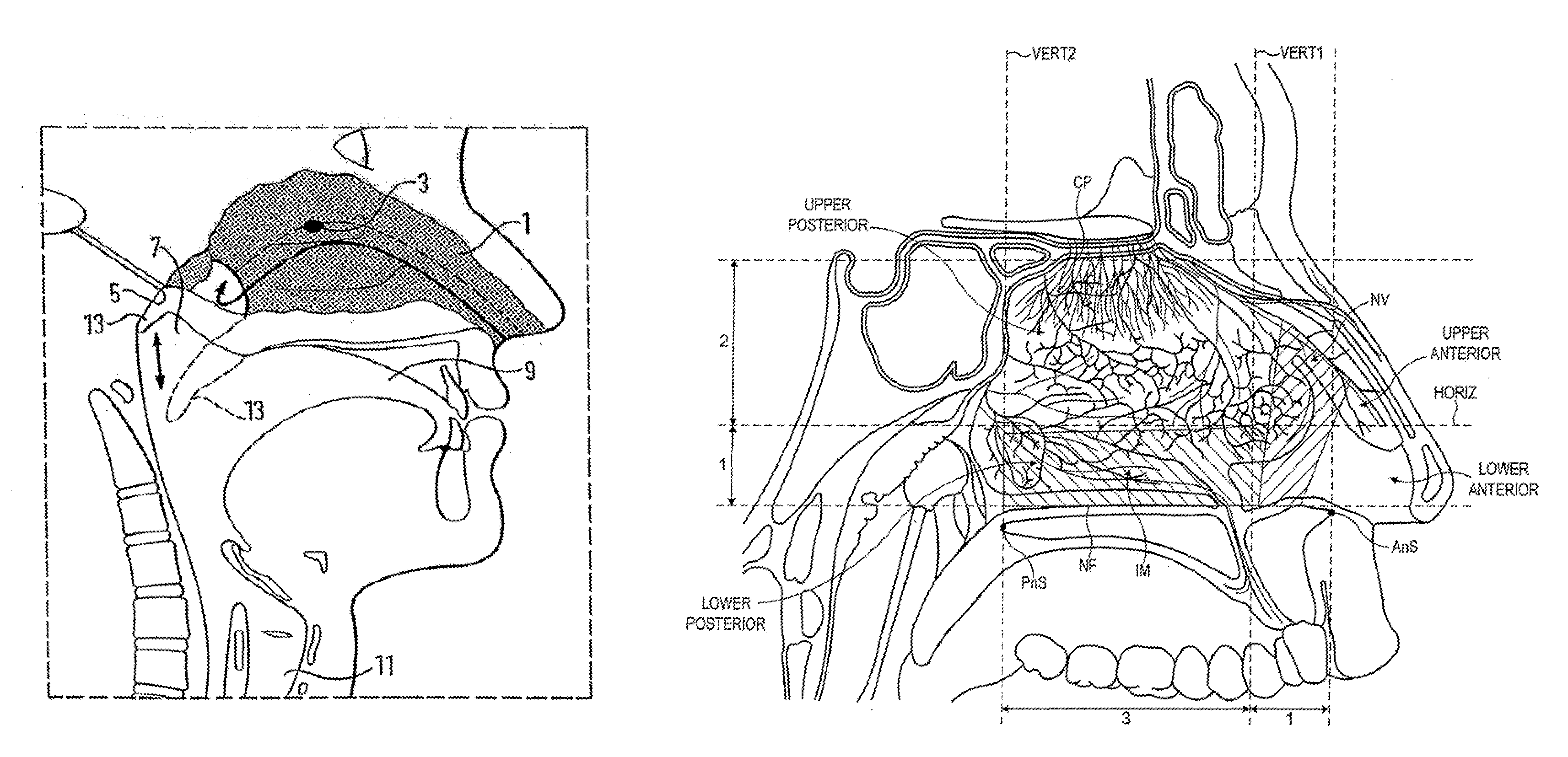
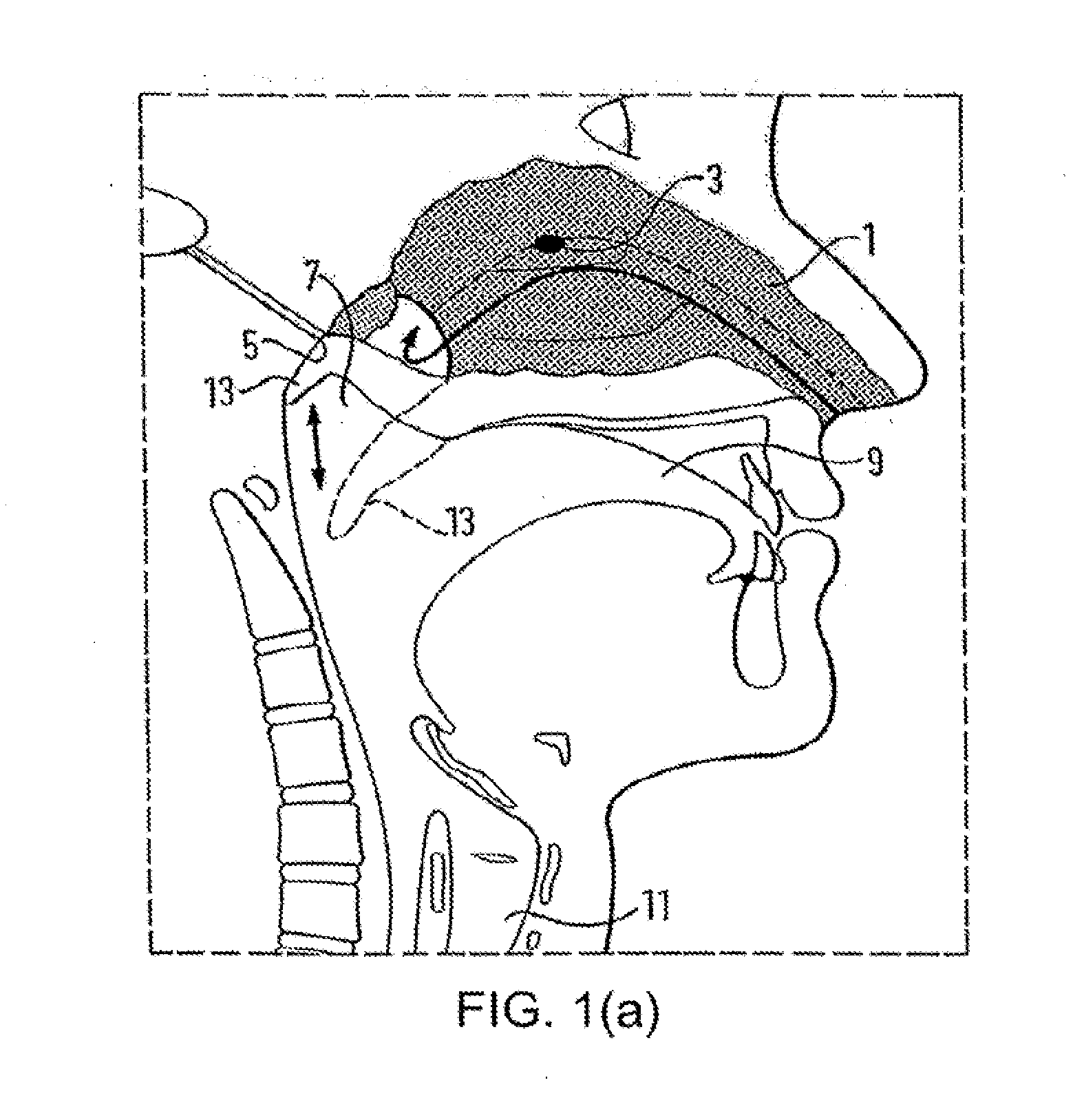
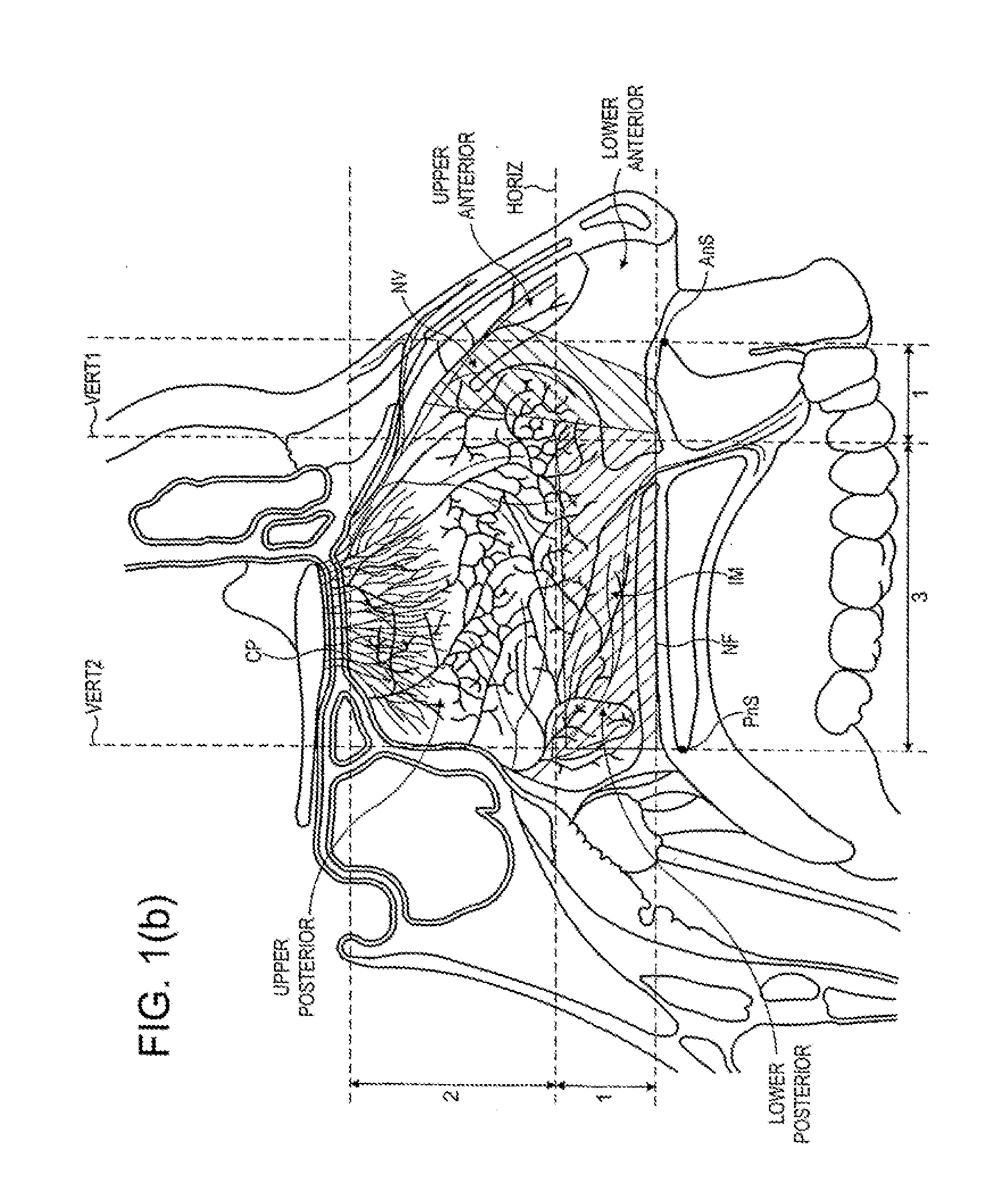
Nasal Administration
a technology of nasal administration and substances, applied in the direction of electrochemical generators, teeth capping, cell components, etc., can solve problems such as unwanted effects, and achieve the effects of increasing the delivery of substances, increasing the uptake of substances into the cns, and increasing the cns
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example # 1
Example #1
[0168]The purpose of this study was to determine the relative sedative effect of midazolam where intranasally delivered using the novel, bi-directional administration system of the present applicant.
[0169]In this study, twelve healthy subjects, 4 male and 8 female, were studied. In separate sessions, the subjects received 3.4 mg of midazolam by one of three different administration systems, these being: an intravenous administration system in which a midazolam formulation was intravenously administered; conventional nasal spray administration system in which a midazolam formulation was conventionally nasally administered using a spray pump as supplied by Ing Erich Pfeiffer GmbH (Radolfsee, Germany) which is specified to generate a liquid spray with a mean particle size of 43 μm, with 100 μl of the formulation being delivered to each nostril; and the bi-directional administration system of the first-described embodiment, and incorporating the same spray pump as the conventi...
example # 2
Example #2
[0190]This study provides for characterization of the deposition as achieved by the nasal administration systems of the above-described study.
[0191]In this study, nine healthy subjects, 4 females and 5 males, were studied.
[0192]In separate sessions, the subjects received a test solution by one of two different nasal administration systems, these corresponding to the nasal administration systems of the above study and being:[0193](i) a conventional nasal spray administration system in which a labeled test solution was conventionally nasally administered using a spray pump as supplied by Ing Erich Pfeiffer GmbH (Radolfsee, Germany) which is specified to generate a liquid spray with a mean particle size of 43 μm, with 100 μl of the test solution being delivered to one nostril; and[0194](ii) the bi-directional administration system of the first-described embodiment, and incorporating the same spray pump as the conventional nasal spray administration system, in which a labeled ...
example # 3
Example #3
[0209]The purpose of this study was to characterize the deposition as achieved by powder aerosol and liquid jet administration systems in accordance with embodiments of the present invention.
[0210]In this study, nine healthy subjects, 4 females and 5 males, were studied.
[0211]In separate sessions, the subjects received a test substance by one of three different nasal administration systems, these being:[0212](i) a conventional nasal spray administration system in which a labeled test solution was conventionally nasally administered using a single-dose spray pump as supplied by Ing Erich Pfeiffer GmbH (Radolfsee, Germany) which is specified to generate a liquid spray with a mean particle size of 43 μm, with 100 μl of the test solution being delivered to one nostril;[0213](ii) the bi-directional administration system of the first-described embodiment where configured to deliver a labeled test powder from a conventional gelatine capsule, with approximately 4 mg of the test po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com