Control of antibody responses to synthetic nanocarriers
a synthetic nanocarrier and antibody technology, applied in the field of synthetic nanocarrier compositions, can solve the problem that prior studies have not addressed the design of synthetic nanocarriers relative to optimizing humoral immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulations of Synthetic Nanocarriers
Materials for Lot #1
[0165]Ovalbumin peptide 323-339 amide acetate salt, was purchased from Bachem Americas Inc. (3132 Kashiwa Street, Torrance Calif. 90505. Part #4065609.) PLGA-R848 conjugate of 75 / 25 lactide / glycolide monomer composition and approximately 4100 Da molecular weight having 5.2% w / w R848 content was synthesized by conjugation of R848 to the terminal-acid of commercially-supplied PLGA via an amide linkage. PLA-PEG-Nicotine with a nicotine-terminated PEG block of 3,500 Da and DL-PLA block of approximately 15,000 Da was synthesized. Polyvinyl alcohol (Mw=9,000-10,000, 80% hydrolyzed) was purchased from SIGMA (Part Number 360627).
Methods for Lot #1
[0166]Solutions were prepared as follows:
[0167]Solution 1: Ovalbumin peptide 323-339 amide acetate salt @ 70 mg / mL was prepared by dissolution in 0.13N hydrochloric acid at room temperature.
[0168]Solution 2: PLGA-R848 @ 75 mg / mL and PLA-PEG-Nicotine @ 25 mg / mL in dichloromethane was prepared...
example 2
Synthetic Nanocarriers with Increased Antigen Increases Antigen-Specific Antibody Generation and Decreases Anti-Carrier Antibody Generation
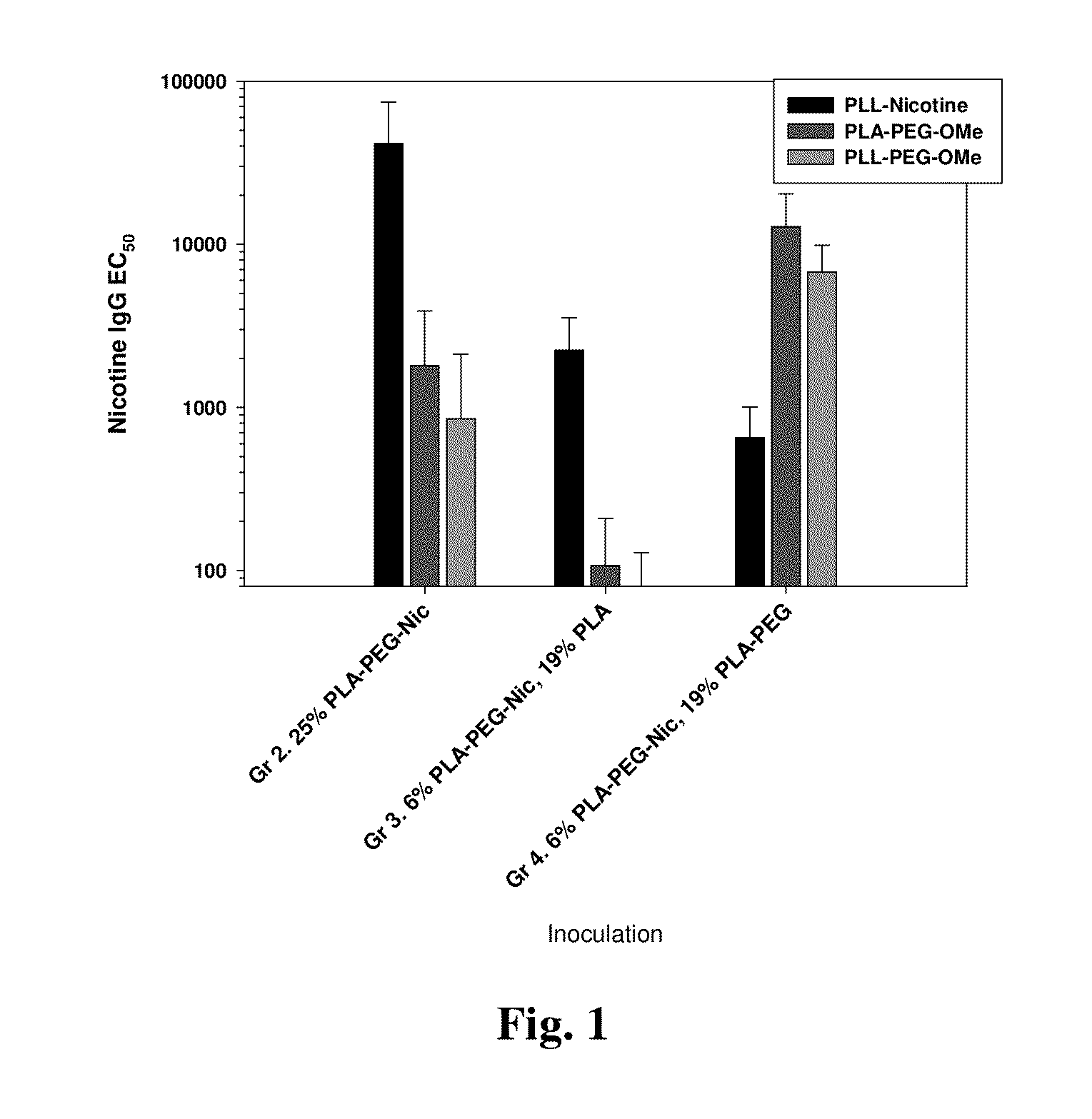
[0193]Mice were inoculated with nicotine-presenting R848-adjuvanted nanocarrier formulations. Groups 2 through 4 were evaluated for antigen-presentation and anti-carrier effect. The nicotine-presenting conjugate in the nanocarrier is a PLA-PEG3.5k-Nicotine construct of ˜15,350 Mw PLA and ˜3500 Mw PEG. The study groups used formulations having varied content of the PLA-PEG3.5k-Nicotine construct, partially-substituting the construct with either a ˜20 k Mw PLA polymer or with a PLA-PEG2k-OMe polymer of ˜18,700 Mw PLA and 2000 Mw PEG. Mice were immunized at days 0, 14, and 28 and serum was collected at days 26 and 40. The formulations are described as tabulated below and the anti-nicotine and resultant anti-PEG antibodies at day 40 are presented in FIG. 1.
TABLE 6Synthetic Nanocarrier Formulationsμg R848 / mgNP releasedPLGA-R848Polymer-AgReplacementR84...
example 3
Synthetic Nanocarriers with Increased Antigen or Increased Polymer Length Decreases Anti-Carrier Antibody Generation
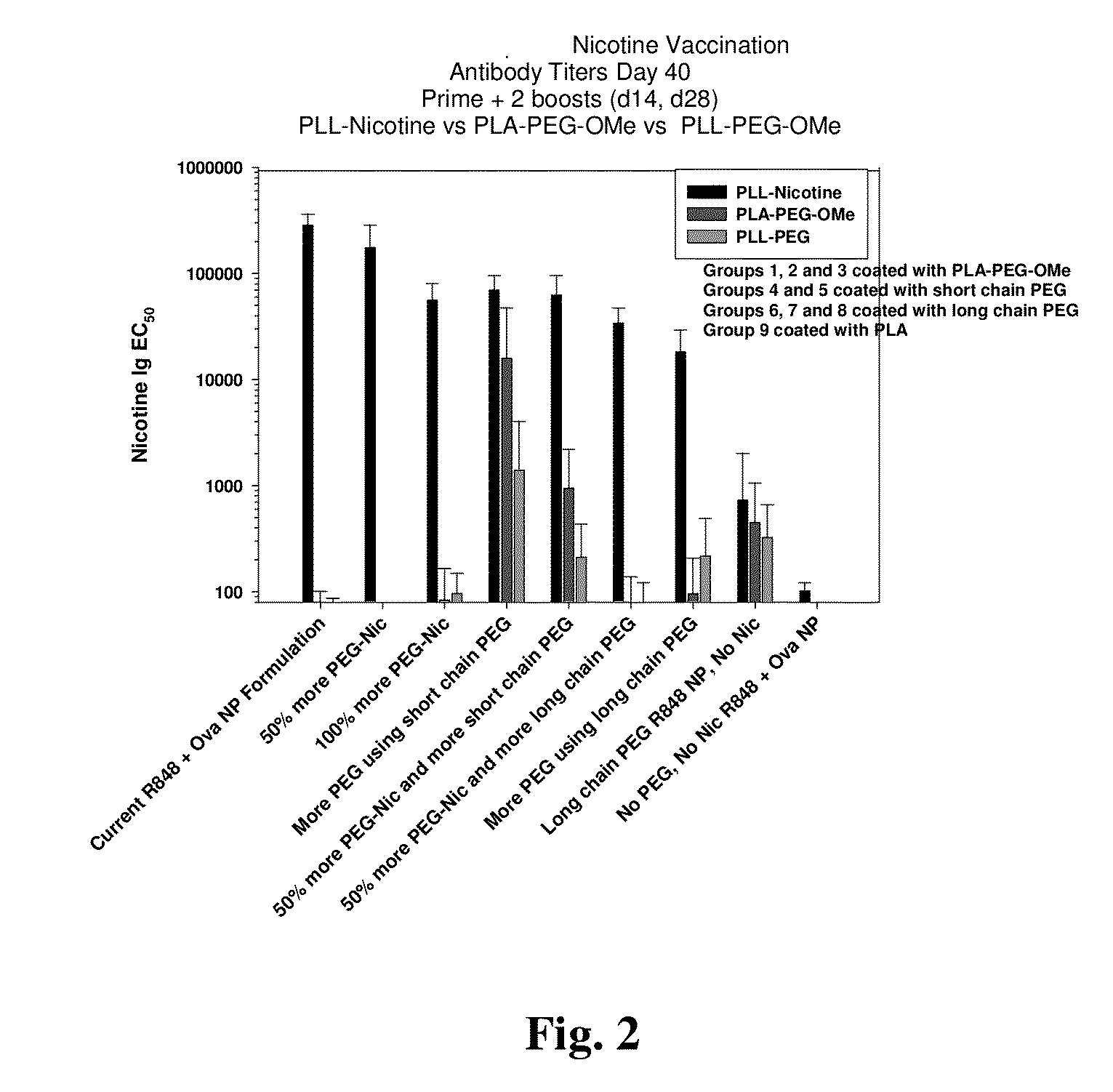
[0196]Mice were inoculated with nicotine-presenting R848-adjuvanted nanocarrier formulations. All formulations were prepared on the same date using a consistent set of solutions and materials. All tested nanocarriers were formulated with a 50% PLGA-R848 polymer content, with the remaining 50% of the composition made up of one or more of the following polymers: PLA-PEG5k-Nicotine, PLA-PEG2k-OMe, PLA-PEG5k-OMe, or PLA.
TABLE 7Synthetic Nanocarrier FormulationsPLA-PEG-PLA-PEG2k-PLA-PEG5k-Ova PeptideR848NicOMeOMePLALoad (%LoadGr.NC Lot #(% w / w)(% w / w)(% w / w)(% w / w)w / w)(% w / w)142500252.04.12537.50012.51.84.436500001.03.9472525001.13.95837.512.5001.04.46937.5012.500.74.17102502500.14.08110050004.6912000500.74.2
[0197]Following a prime and two-boost inoculation schedule, the on-target (anti-nicotine) antibody titers and off-target (anti-PEG) antibody titers were determined by E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com