Anti-human PCSK9 monoclonal antibody
A monoclonal antibody and antibody technology, applied in the treatment of diseases mediated by human PCSK9, in the field of human-derived functional anti-human PCSK9 monoclonal antibodies, can solve the problems of patients' intolerance and failure of blood lipid levels to reach the standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of various recombinant proteins.
[0030] A variety of different recombinant proteins are used in the preparation of anti-PCSK9 monoclonal antibodies, including human PCSK9 (huPCSK9, SeqIDNo: 1), mouse PCSK9 (moPCSK9, SeqIDNo: 2) and macaque PCSK9 (mmPCSK9, SeqIDNo: 3), Human LDLR extracellular region (EGF-AB, SeqIDNo:4), and recombinant antibody. These proteins have a large number of post-translational modifications (such as: glycosylation or disulfide bonds, etc.), so the use of mammalian cell expression systems will be more conducive to maintaining the structure and function of recombinant proteins. In addition, for the convenience of purification, a His-tag (SeqIDNo:5) or the Fc fragment of a mouse antibody (mFc, SeqIDNo:6) was added to the C-terminus of the non-antibody recombinant protein. When preparing recombinant antibodies, the heavy chain constant region of the antibody can be of IgG1 subtype (SeqIDNo: 7), IgG2 subtype (SeqIDNo: 8) o...
Embodiment 2
[0033] Example 2: Using phage display antibody library technology to screen and optimize anti-human PCSK9 monoclonal antibody.
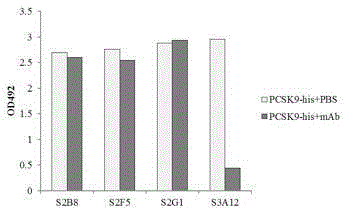
[0034] Using the prepared recombinant huPCSK9-his (hereinafter abbreviated as PCSK9-His) as the antigen, refer to the literature (anti-human IL-17 monoclonal antibody, patent application number 201510097117.0), and use the solid-phase screening strategy to screen the phages presenting the human single-chain antibody library , obtained multiple human antibodies with different sequences but specific binding to human PCSK9, including clones: S2B8 (SeqIDNo: 12), S2F5 (SeqIDNo: 13), S2G1 (SeqIDNo: 14), S3A12 (SeqIDNo: 15). Among them, the recombinant full antibody S3A12 can effectively inhibit the binding of PCSK9 to the EGF-AB domain of the extracellular region of recombinant human LDLR ( figure 1 ).
[0035] Then referring to the literature (anti-human IL-17 monoclonal antibody, patent application number 201510097117.0), the antibody was subjected to...
Embodiment 3
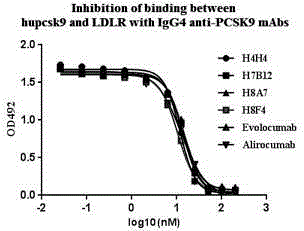
[0037] Example 3: BIAcore3000 measures the affinity of whole antibodies.
[0038] Aminecoulingkit and humanantibodycapturekit as well as CM5 chip and pH7.4 10×HBS-EP were purchased from GE Healthcare.
[0039] According to the instructions in the kit, anti-human F C The antibody of the segment is coupled to the surface of the chip CM5, and the antibody protein is diluted to an appropriate concentration to ensure that about 200RU of the antibody is captured by the anti-human Fc antibody. Set a series of concentration gradients (ex.100nm, 50nm, 25nm, 12.5nm, 6.25nm, 3.125nm, 1.5625nm, 0nm) of huPCSK9 to flow through the surface of the stationary phase, and measure the affinity of each monoclonal antibody, and use the same method to determine The affinity of evolocumab (SeqIDNo:21, SeqIDNo:22) and alirocumab (SeqIDNo:23, SeqIDNo:24) was used as a control. The results are shown in Table 2.
[0040] Antibody Kon (1 / MS) Koff(1 / S) KD H4H4-IgG4 7.14E+05 4.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com