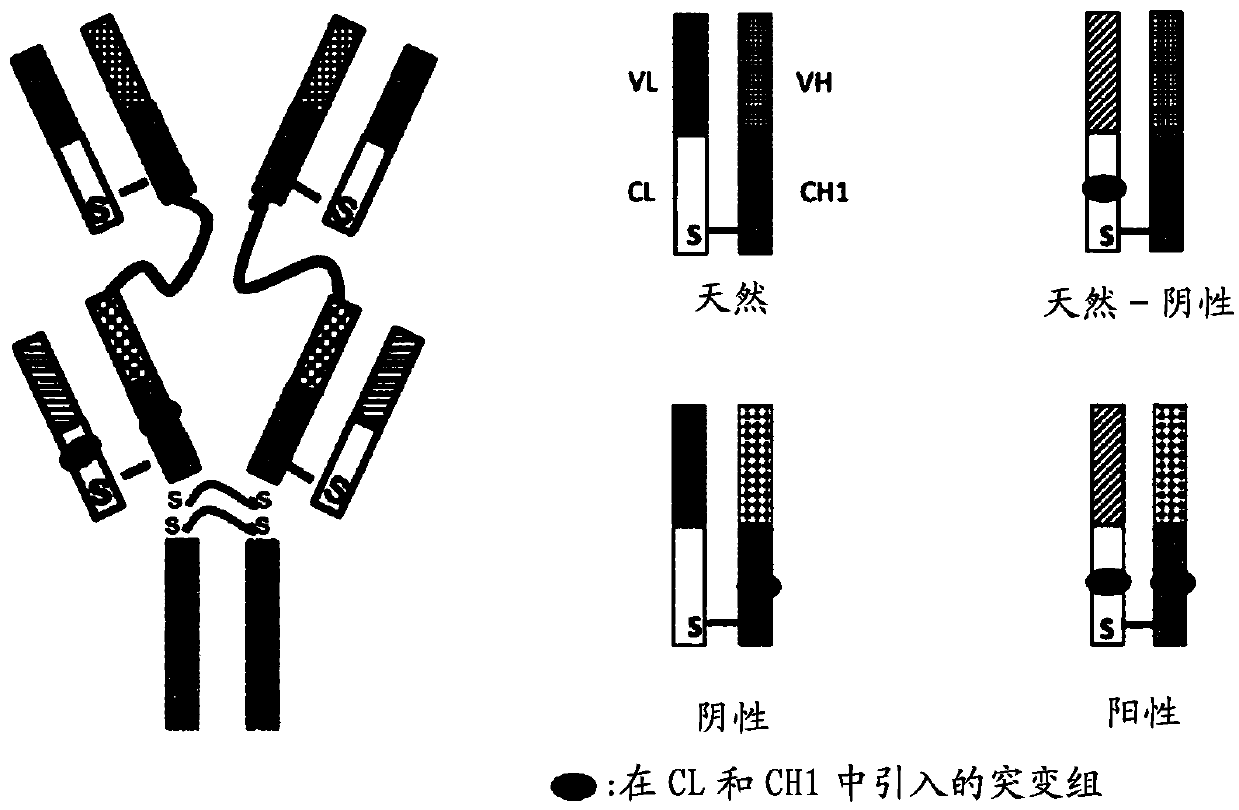
Bispecific antibodies targeting EGFR and HER2
A bispecific antibody, antibody technology, applied in the direction of antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, specific peptide, etc., can solve the problem that the full efficacy of EGFR and HER2 inhibition has not yet been achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
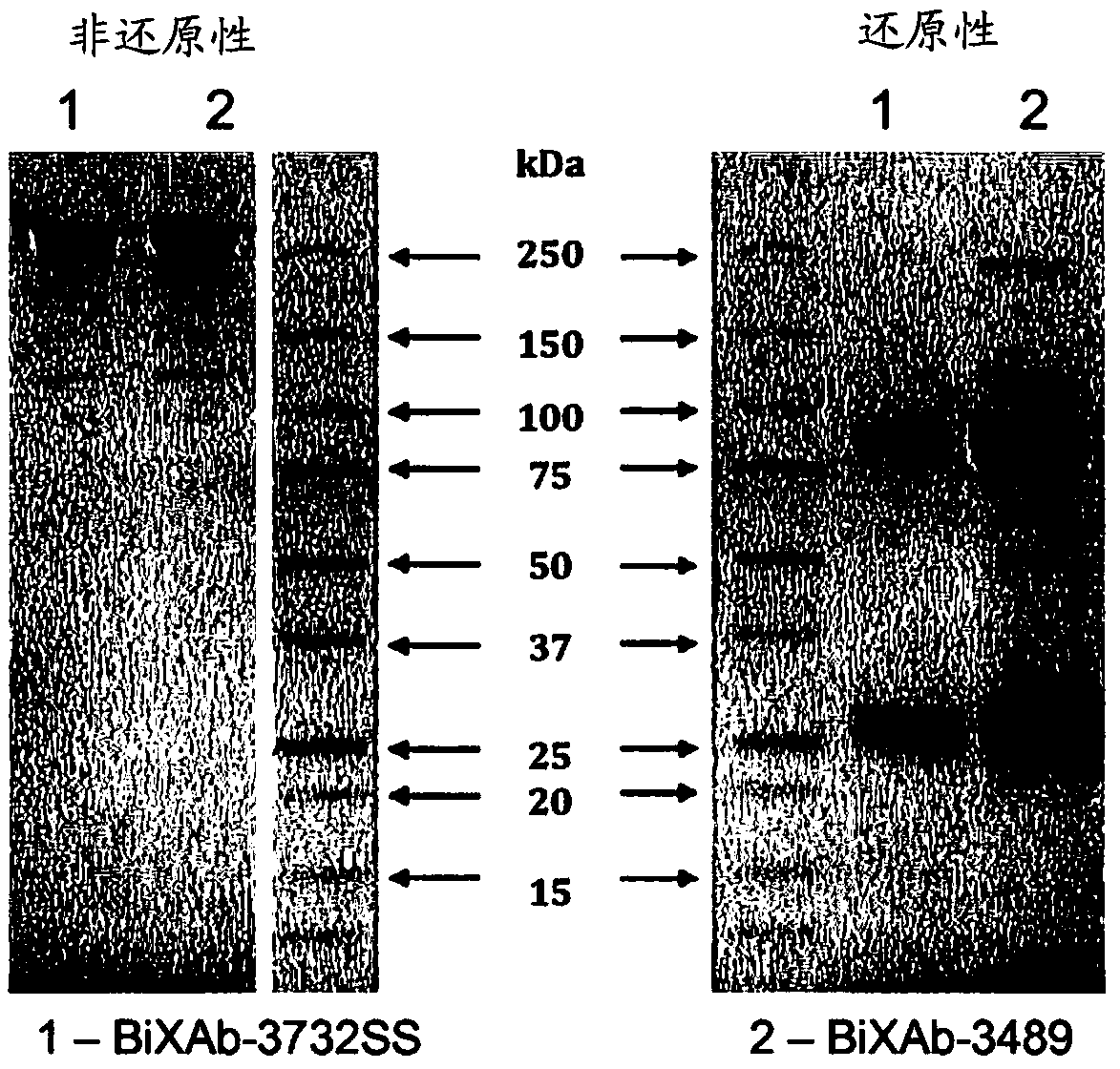
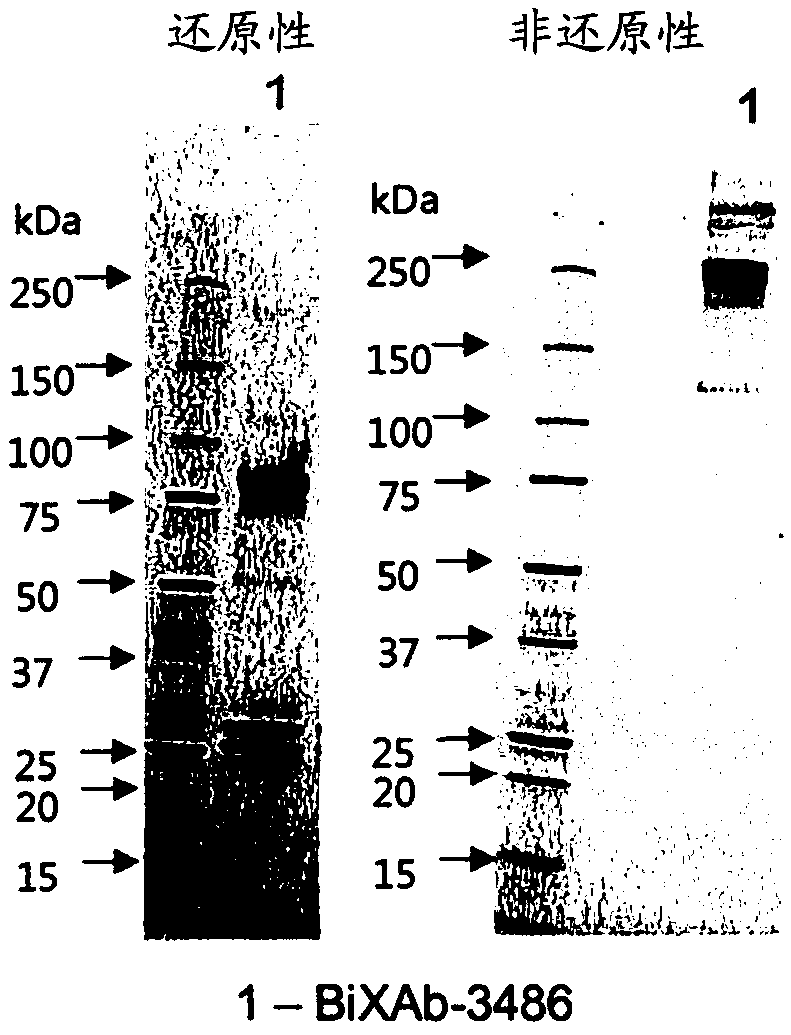
[0511] Example 1: Preparation of bispecific antibodies BiXAb-3486, BiXAb-3489 and BiXAb-3732SS of the present invention
[0512] gene synthesis
[0513] After codon optimization for mammalian expression, anti-HER2 (trastuzumab, clone humAb4D5-8) (Carter P., Presta L., Gorman C.M., Ridgway J.B., Henner D., Wong W.L., Rowland A.M., Kotts C., Carver M.E., Shepard H.M. (1992) Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U SA.15, 4285-4289) and anti-EGFR (cetuximab) The amino acid sequence of (Humblet Y. (2004). Cetuximab: an IgG1 monoclonal antibody for the treatment of epidermal growth factor receptor expressing tumors. Expert Opin Pharmacother 5: 1621-1633) was used to design the DNA sequence. For the heavy chain, DNA encoding the signal peptide, the variable region of Fab1 and the constant CH1 domain followed by a pseudo-hinge junction and the variable region and constant CH1 domain of Fab2 were synthesized by GeneScript with flankin...
Embodiment 2
[0531] Example 2: Preparation of the bispecific antibody BiXAb-E06528 of the present invention
[0532] gene synthesis
[0533] After codon optimization for mammalian expression, the amino acid sequences of anti-EGFR (cetuximab) and anti-HER2 (trastuzumab) were used to design the DNA sequence using the GeneScript program. These antibodies are referred to as "parental" anti-EGFR and "parental" anti-HER2 mAbs.
[0534] The DNA construct of the heavy chain was designed in the following way: signal peptide, followed by a sequence consisting of the variable region, followed by the constant CH1 domain of Fab1 (anti-HER2), followed by the AP linker, followed by the variable region , followed by the constant CH1 domain of Fab2 (anti-EGFR), where a Thr to Glu mutation was introduced at Kabat position 192; flanking sequences for restriction enzyme digestion were introduced at both ends of the heavy chain DNA construct. The DNA construct for the light chain was designed in the follow...
Embodiment 3
[0546] Example 3: Characterization by Differential Scanning Calorimetry
[0547] The thermal stability of BiXAb-3489, parental anti-HER2 mAb and parental anti-EGFR mAb was compared using differential scanning calorimetry (DSC). Differential scanning calorimetry experiments were performed using a Microcal™ VP-capillary DSC system (Malvern Instruments).
[0548] All samples were centrifuged (20,000 x g, 5 min, 4°C) and their protein content was quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific) with IgG analysis program before DSC analysis. For assays, all samples were diluted in PBS to a final concentration of 1 mg / mL.
[0549] The pre-equilibration time was 3 min, and the resulting thermograms were collected between 20 and 110°C, at a scan rate of 60°C / h, with a filtration period of 25 seconds and media feedback. Prior to sample analysis, 5 buffer / buffer scans were measured to stabilize the instrument, with buffer / buffer scans performed between each pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com