Anti-HER2 dual-targeting antibodies, and preparation method and application thereof
A dual-targeted antibody, antibody technology, applied in the biological field, can solve the problem of difficult to kill tumors and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
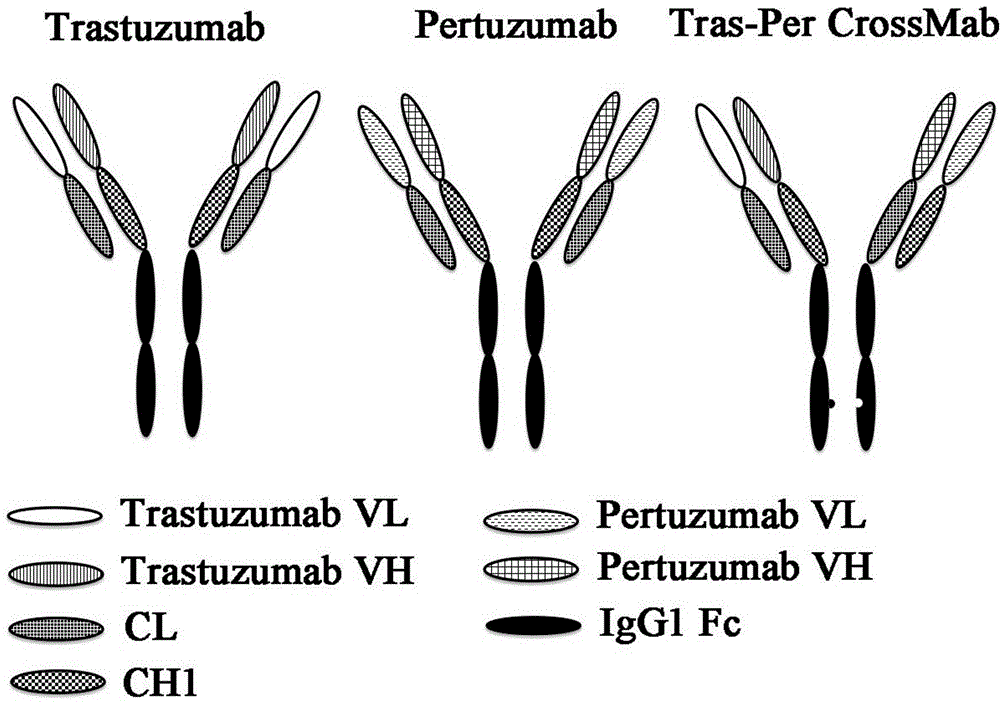
[0061] Example 1. Trastuzumab and Pertuzumab Antibody heavy chain variable region, Pertuzumab Cloning of light chain variable region gene
[0062] Use Trastuzumab or Pertuzumab antibody heavy chain as a template to clone Trastuzumab and Pertuzumab heavy chain variable region genes and Pertuzumab light chain variable region gene by PCR respectively. The reaction conditions are: 95°C for 15 minutes; 94°C for 50 seconds, 58°C for 50 seconds , 72°C for 50 seconds, 30 cycles; 72°C for 10 minutes to obtain the PCR products Trastuzumab HV, Pertuzumab HV and Pertuzumab LV. Antibody signal peptide amino acid sequence MGWSCIILFLVATATGVHS. The correct cloned fragments were obtained by sequencing. SEQ ID NO: 2 shows the amino acid sequence of Trastuzumab heavy chain variable region, its nucleotide sequence is SEQ ID NO: 1; SEQ ID NO: 3 is the nucleotide sequence of Pertuzumab heavy chain variable region, its amino acid sequence is SEQ ID NO: 4; SEQ ID NO: 6 shows the amino acid sequ...
Embodiment 2
[0063] Example 2. Antibody light chain constant region, heavy chain constant region 1, Fc area cloning
[0064] Lymphocyte separation medium (product of Dingguo Biotechnology Development Co., Ltd.) was used to separate healthy human lymphocytes, and total RNA was extracted with Trizol reagent (product of Invitrogen Company). According to the literature (Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Hieter PA, Max EE, Seidman JG, Maizel JV Jr, Leder P. Cell. 1980 Nov;22(1 Pt 1):197-207.) and literature (The nucleotide sequence of a human immunoglobulin C gamma1 gene. Ellison JW, Berson BJ, Hood LE. Nucleic Acids Res. 1982 Jul 10;10(13):4071-9.) RT-PCR was used to amplify the antibody light chain constant region, heavy chain constant region CH1 and Fc region genes. The PCR product was purified and recovered by agarose gel electrophoresis and cloned into the pGEM-T vector. After sequencing verification, ...
Embodiment 3
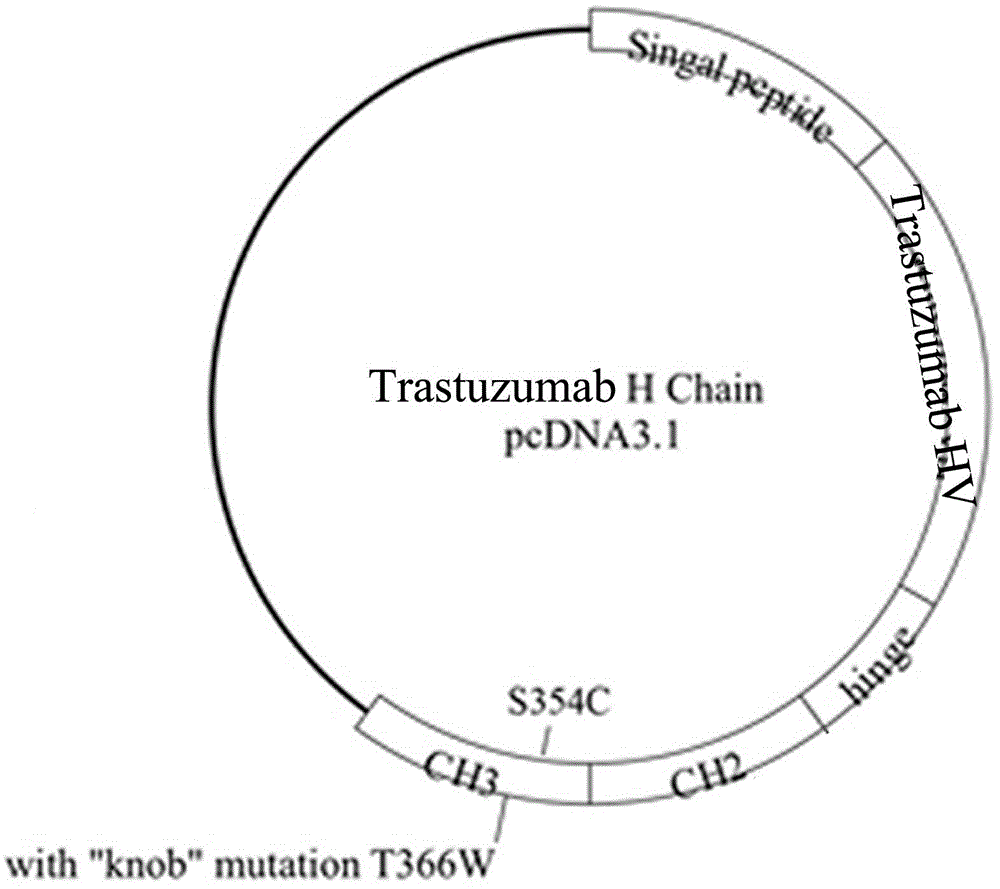
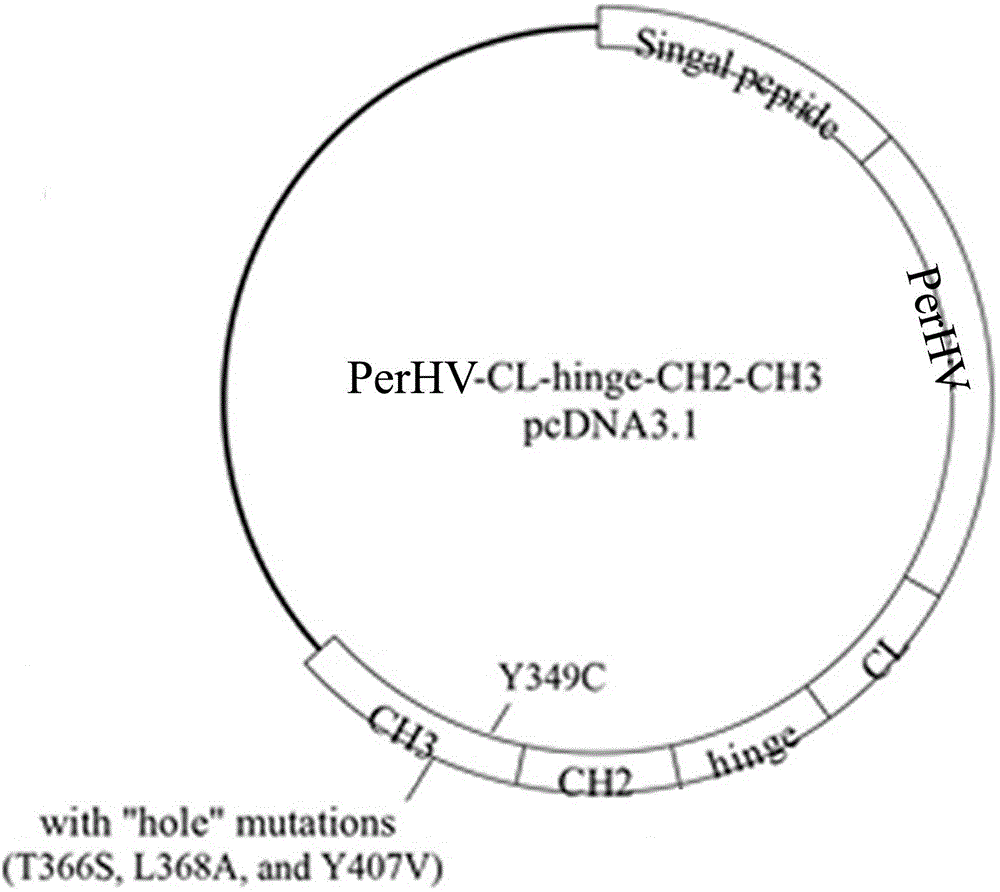
[0065] Example 3. Antibody Fc Area knob Construction of mutants
[0066] The Fc region of the antibody obtained in Example 2 was introduced into the mutation point by overlapping PCR method: T366W, S354C (Schaefer WW, Regula JTJ, Böhner MM, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011;108(27):11187–11192.). The PCR product was purified and recovered by agarose gel electrophoresis and cloned into the pGEM-T vector. After sequencing verification, it was confirmed that the correct clone was obtained. SEQ ID NO:14 shows the amino acid sequence of Fc-knob, and its nucleotide sequence is SEQ ID NO:13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com