Rabbit bordetella bronchiseptica subunit vaccine
A technology of Bordetella sanguinis and subunit vaccines, which can be applied in vaccines, veterinary vaccines, antibacterial drugs, etc., can solve the problems of bacterial toxin residues and immunization rabbits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
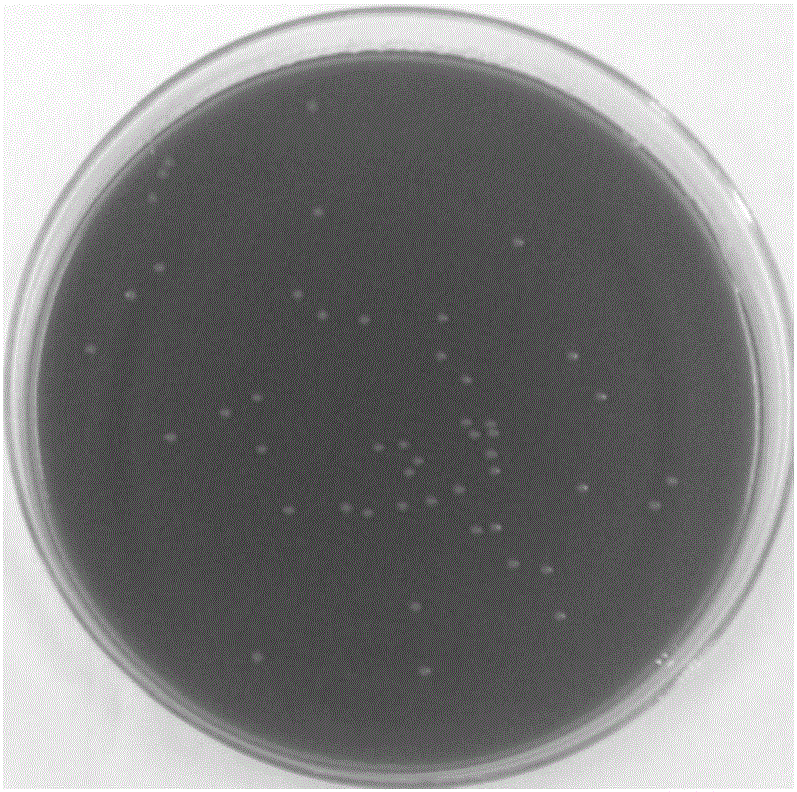
[0014] Example 1: Isolation and cultivation of Bordetella bronchiseptica in rabbits
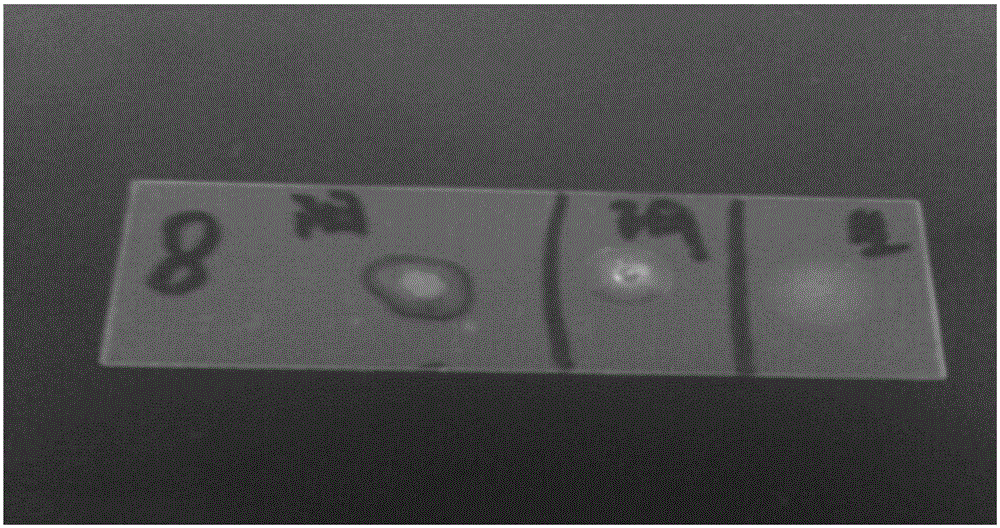
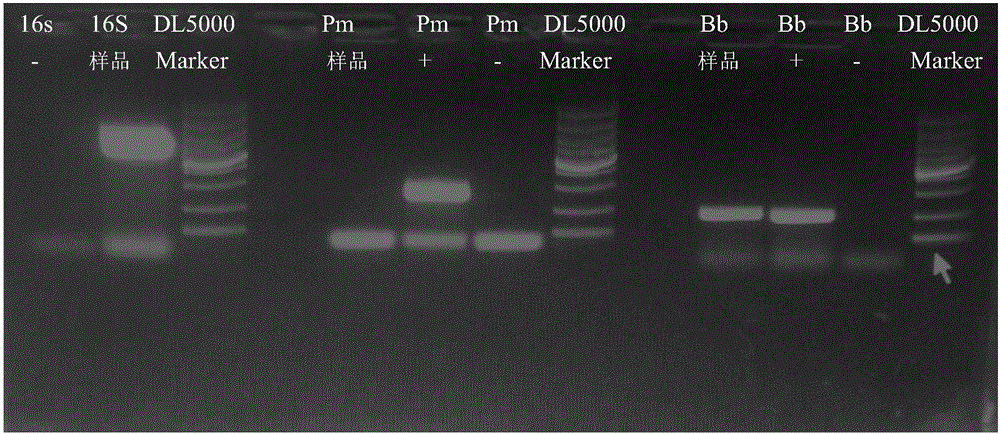
[0015] A batch of 1.5-3.0 kg rabbits were purchased from a rabbit farm in Weifang, Shandong Province for the test of swine fever spleen leaching vaccine. The disease was characterized by acute onset, rapid death, mouth and nose bleeding, lung bleeding, and congestion. According to the incidence and death of rabbits Clinical manifestations, autopsy lesions, preliminarily suspected infection of Pasteurella rabbit or Bordetella bronchiseptica rabbit, so the lung tissue was taken to isolate and culture the bacteria, and the slide agglutination test was carried out for verification; Pasteurella (Pm) gene or Bordetella (Bb) gene was amplified by PCR, cloned, and sequenced. It was confirmed that the batch of rabbits died of infection caused by Bordetella bronchiseptica.
[0016] 1.1 Incidence situation A batch of 1.5-3.0kg rabbits was purchased from Weifang, Shandong Province for the temperature mea...
Embodiment 2
[0030] The purification of the protein of embodiment 2 rabbit Bordetella bronchiseptica (QDBb01 strain)
[0031] The isolated, identified and preserved Bordetella bronchiseptica (QDBb01 strain) was cultured in 3% TSB, and the bacteria were collected at 4000r / min, and the bacteria were resuspended with PH7.2 Tris-Hcl, and the resuspended bacteria were ultrasonically broken. Work for 1s, the interval time is 3s, the whole time is 12min, a total of 180 times. 4000r / min low-speed centrifugation to remove unbroken bacterial cells and heavier bacterial intracellular organelles, take the supernatant at 100000r / min, ultracentrifuge for 20min, and remove the supernatant. Dissolve the precipitate containing outer membrane protein with Tris-Hcl buffer containing 2% Triton X-100, incubate at room temperature for 1 hour to remove inner membrane protein, then perform ultracentrifugation at 100,000 r / min for 30 minutes, resuspend the precipitate with buffer, and use The semi-permeable membr...
Embodiment 3
[0032] Embodiment 3: Rabbit Bordetella bronchiseptica (QDBb01 strain) subunit vaccine preparation
[0033]Purified Bordetella bronchiseptica protein is carried out in absolute quantification by spectrophotometry according to the following steps: (1) add 0.15ml of PBS for protein measurement and 2.85ml of Coomassie Brilliant Blue staining solution for protein quantification in a cuvette As a contrast, carry out zero adjustment; (2) add 0.14ml of PBS for the protein to be tested, add 0.01ml of the protein solution to be tested and 2.85ml of Coomassie brilliant blue staining solution for protein quantification, measure the absorbance value y in the cuvette to be 0.204, pass The formula y=0.152x+0.068 gives the protein concentration x=0.83 mg / ml, which is 895 μg / ml. The above reagents for protein measurement were purchased from TIANGEN Company. Dilute the purified protein solution to 150ug / ml, mix the vaccine with aluminum hydroxide gel adjuvant at a volume ratio of 1:4, and prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com