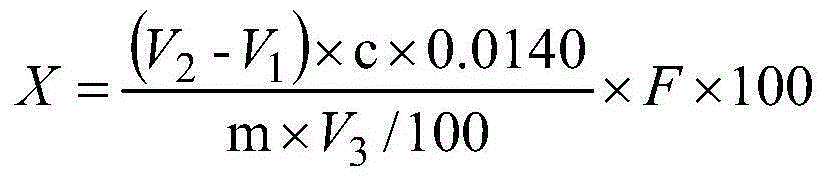Patents
Literature
30 results about "Protein measurement" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Direct Assay of Skin Protein in Skin Samples Removed by Tape Stripping
InactiveUS20080188387A1Cost effectiveImprove throughputCosmetic preparationsMake-upSAA proteinMedicine
The present invention provides for a method of measuring the amount of skin removed by tape stripping. In one aspect of the invention, the invention provides a method for the direct assay of protein in skin samples removed by tape stripping, with a view to combining the protein measurement obtained with a corresponding skin cholesterol measurement to identify individuals at risk of having atherosclerosis as well as those at risk of developing atherosclerosis and similar diseases associated with and attributable to high cholesterol levels. Moreover, the present invention allows a comparative measurement of the amount of skin removed by tape stripping that does not rely solely on the area of the sample removed. Additionally, in one aspect of the invention, the method of this invention can allow relative levels of skin cholesterol to be compared based on the relative amounts of skin removed.
Owner:MIRACULINS
Method for both time and frequency domain protein measurements
InactiveUS20060289785A1Raise the potentialHigh voltage switchingRaman/scattering spectroscopyPhotometryTime domainCapacitance
The invention relates to methods and devices for luminescent (e.g., fluorometric) measurement. The disclosure includes frequency domain and single photon counting methods and utilizes low capacitance semiconductor light emitting devices.
Owner:HORIBA JOBIN YVON
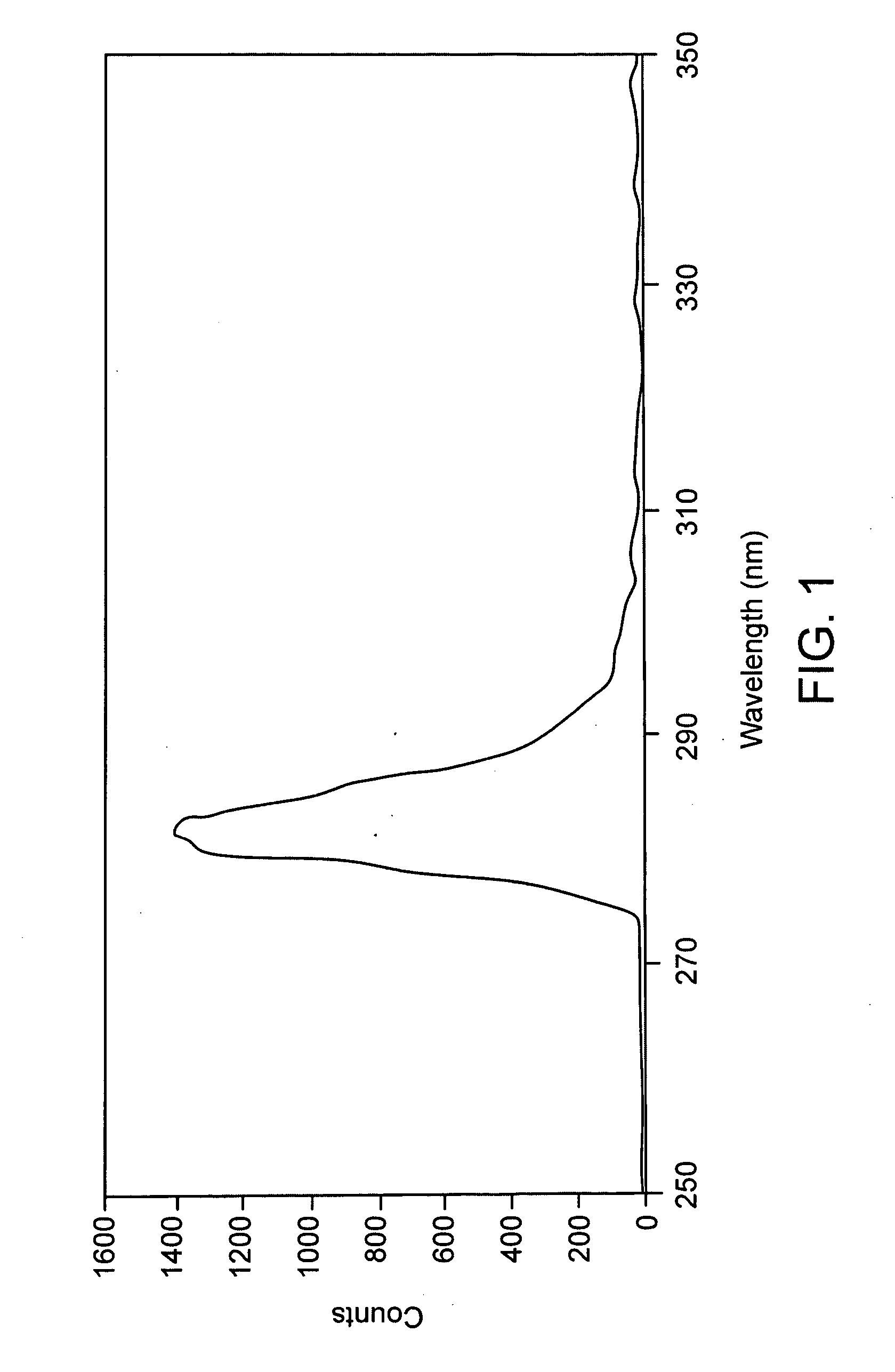
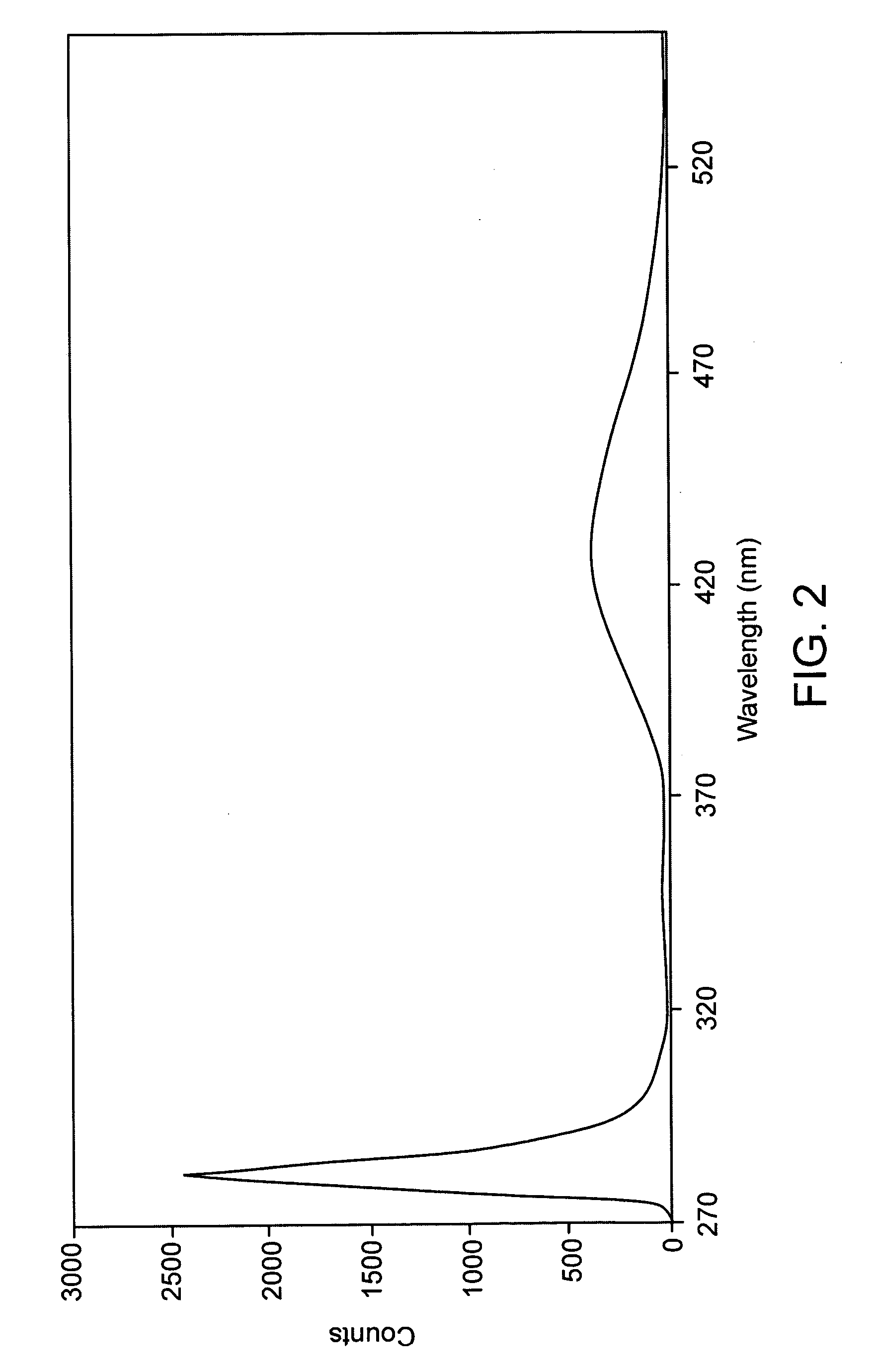
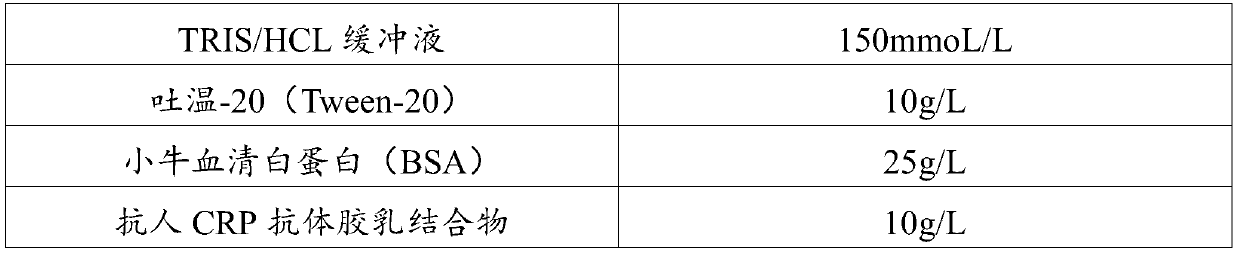
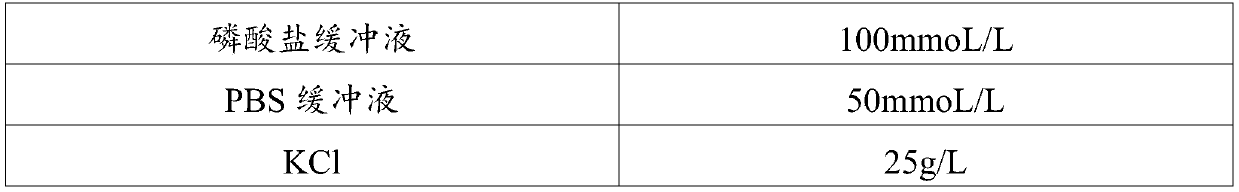
CRP (C-Reactive Protein) assay kit, and preparation method and application thereof
ActiveCN110646618ANo increase in R&D costsImprove detection accuracyMaterial analysis by observing effect on chemical indicatorDisease diagnosisActive agentSurface-active agents
The invention relates to a CRP (C-Reactive Protein) assay kit, and a preparation method and application thereof, and belongs to the technical field of IVD reagents (In Vitro Diagnostic Reagents). TheCRP assay kit provided by the invention comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises buffer solutions, inorganic salts, surfactants, stabilizing agents and preservatives;and the reagent R2 comprises buffer solutions, surfactants, sealing agents, anti-human CRP antibody latex conjugates and preservatives. The CRP assay kit provided by the invention has the advantages that on the basis of the existing latex immunoturbidimetry, a reaction system is optimized by a specific preparation method through an anti-freezing agent; the detection accuracy, the analysis sensitivity and precision of tested reagents are further improved; the research and development cost of the reagents are not improved; and the CRP assay kit can easily adapt to the development of the modern clinical laboratory medicine.
Owner:广州市伊川生物科技有限公司
Determination method for absorption and transportation amount of six components in rhizoma bletillae in Caco-2 cell model
ActiveCN105954411AEvaluation of in vivo absorption propertiesComponent separationInternal standardIn vivo absorption
The invention discloses a determination method for the absorption and transportation amount of six components in rhizoma bletillae in a Caco-2 cell model. The method comprises the steps of: preparing a rhizoma bletillae extract solution, a standard solution serving as a reference substance and an internal standard solution; establishing a human-derived colon adenocarcinoma cell line Caco-2 cell model; preparing a cell suspension through a Caco-2 cell model; determining the content of the six components by UPLC-MS / MS; determining the total protein content according to a Coomassie brilliant blue dye liquor protein determination kit method, and calculating the cell uptake X=the total protein of a to-be-determined substance. The invention adopts UPLC-MS / MS to establish the analysis method for the 6 components in a rhizoma bletillae extract, determines the influence of the rhizoma bletillae extract to absorption and uptake of Caco-2 cells under the conditions of time, concentration, temperature, pH and P-gh inhibitors, preliminarily evaluates the in vivo absorption characteristics of the rhizoma bletillae extract, and provides scientific basis for the research and development of the oral preparation of the rhizoma bletillae extract.
Owner:GUIZHOU MEDICAL UNIV
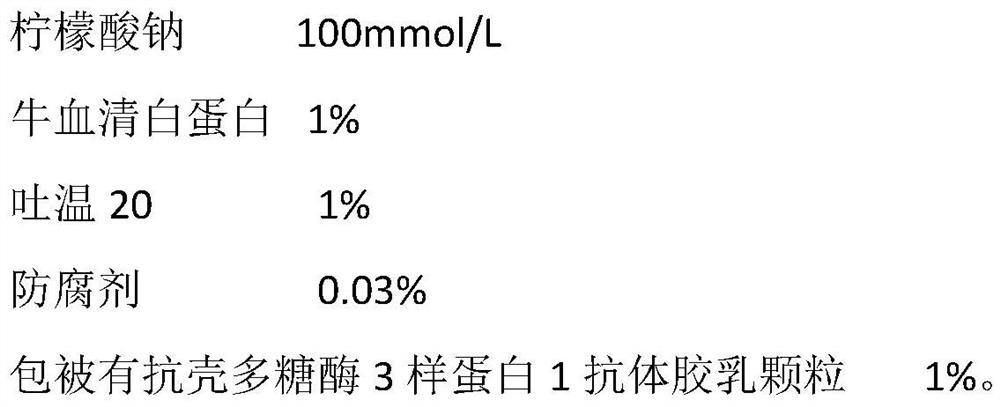
Chitosanase 3-like protein 1 determination kit
PendingCN112526134AHigh sensitivityIncreased sensitivityMaterial analysisAntiendomysial antibodiesBovine serum albumin
The invention discloses a chitosanase 3-like protein 1 determination kit, and relates to the technical field of biological analysis. The chitosanase 3-like protein 1 determination kit comprises a reagent R1 and a reagent R2; the reagent R1 comprises 50 to 300 mmol / L of sodium citrate, 0.05 to 0.5 mol / L of sodium chloride and 0.02 to 5% of a preservative, and the reagent R2 comprises 50 to 300 mmol / L of sodium citrate, 0.5 to 5% of bovine serum albumin, 0.2 to 2% of Tween 20, 0.02 to 5% of a preservative and 0.5 to 5% of latex particles coated with an anti-chitosanase 3-like protein 1 antibody.The kit provided by the invention has the advantages of high sensitivity, strong specificity, simple operation and the like, can be applied to various full-automatic biochemical analyzers for large-batch determination, is suitable for clinical popularization, and reduces the hospital detection cost.
Owner:BEIJING 3S CENTURY TECH CORP
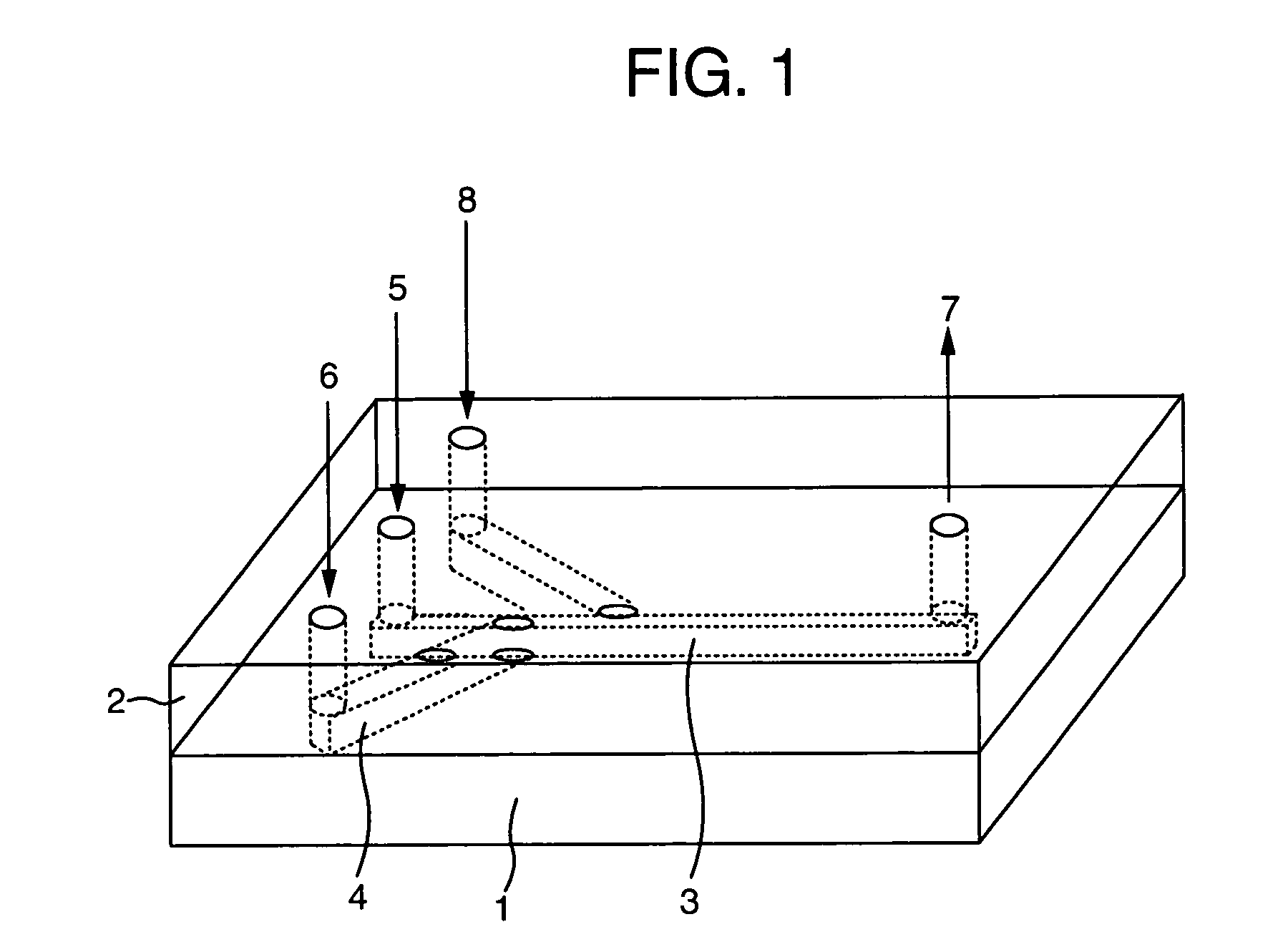
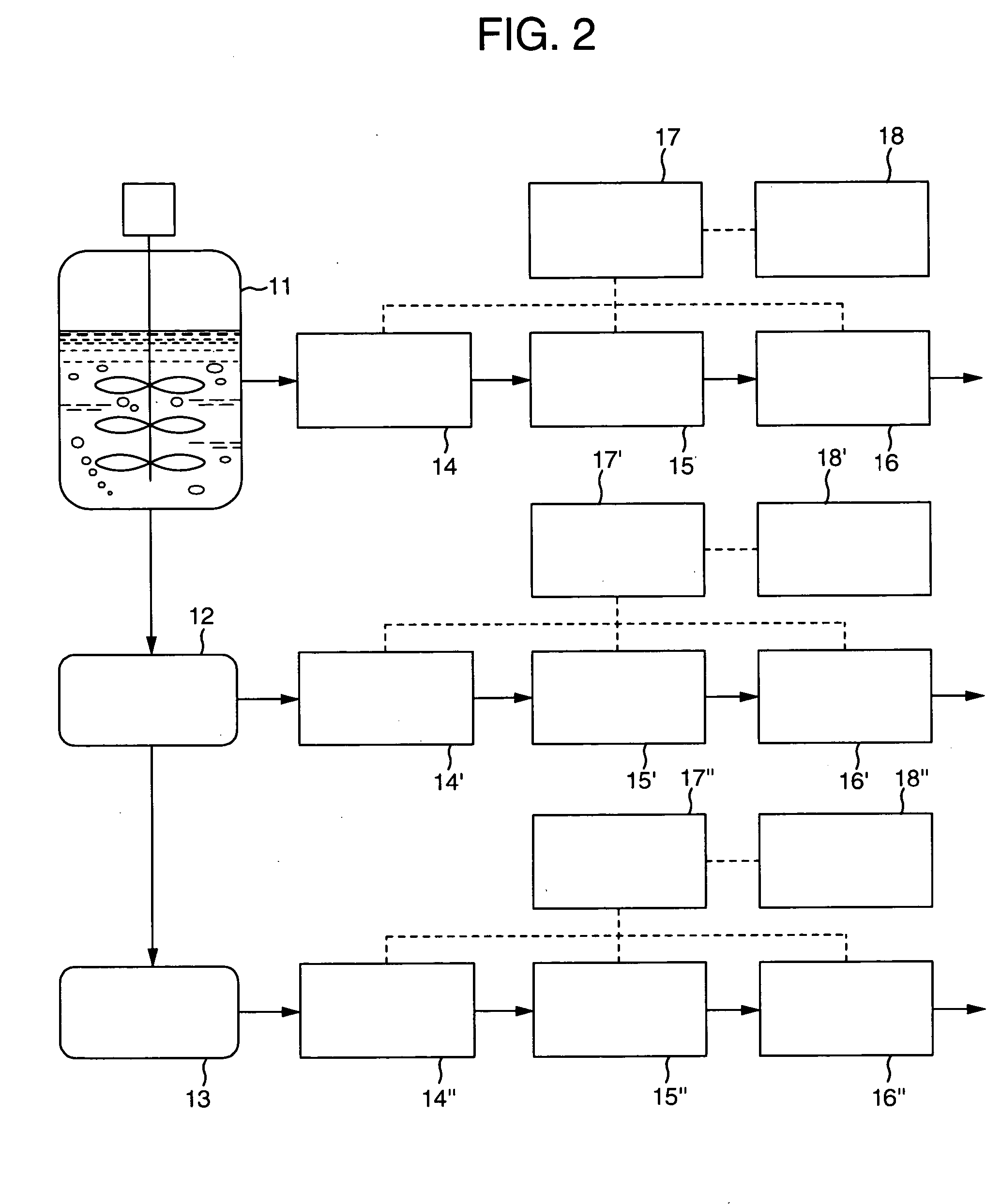
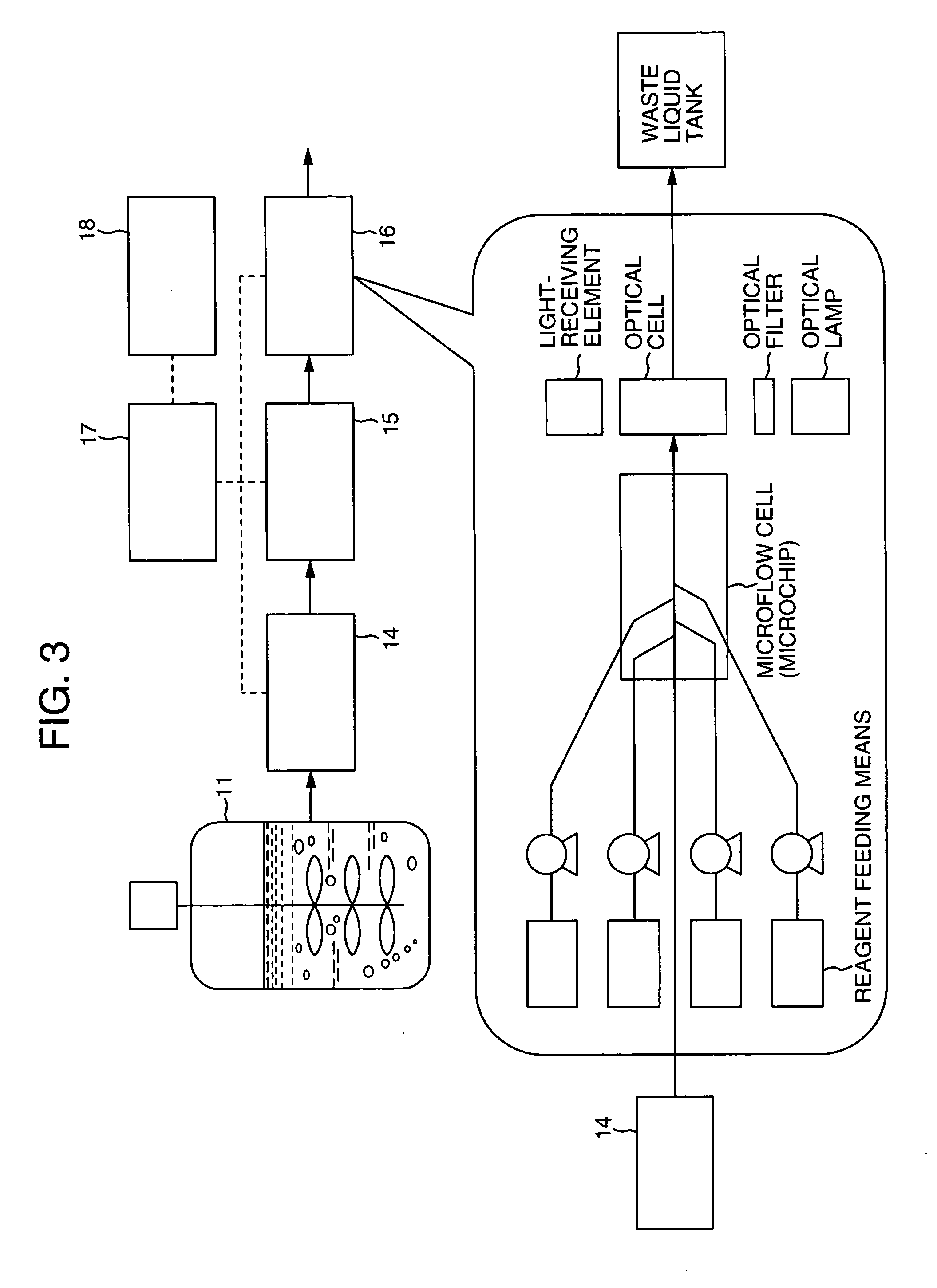
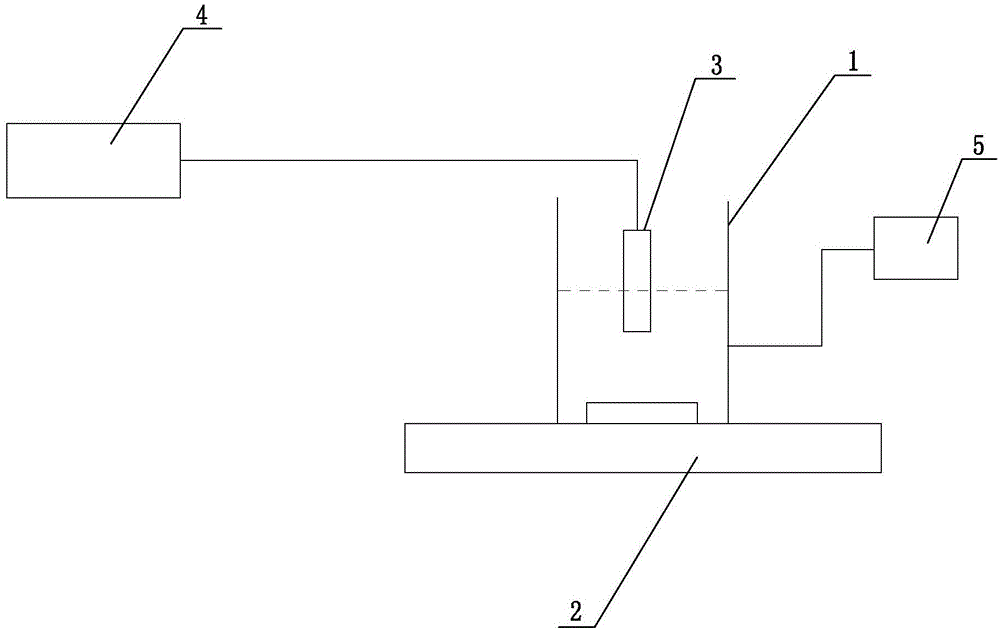
Protein measurement method in protein production plant by cell culture and apparatus thereof
InactiveUS20040157270A1Bioreactor/fermenter combinationsBiological substance pretreatmentsMeasurement deviceAutomatic control
An automatic protein quantitative measurement apparatus having a reduced burden on an analysis operator, being free from bacterial contamination in each process device during collection of a sample liquid, having reduced measurement time and being capable of downsizing is provided. The automatic protein quantitative measurement apparatus has a sampling unit for automatically collecting a sample liquid online from at least one operation process of a cell culture step (wherein an antibody or other proteins are produced and secreted into the culture medium) and a separation step or a culture product purification step in a plant for culturing biological cells to produce a protein, a preprocessing unit for performing at least one of dilution and filtration of the sample liquid to adjust the liquid composition, a reaction unit having a cross-sectional area of 0.04 mm<2 >or less and quantitatively measuring a protein contained in the adjusted liquid using an antigen-antibody reaction, a measurement unit for quantitatively measuring the protein according to the result of the reaction, a control unit for automatically controlling a series of operations of the above units constituting the apparatus, and a recording unit for recording the result of quantitative measurement.
Owner:HITACHI LTD
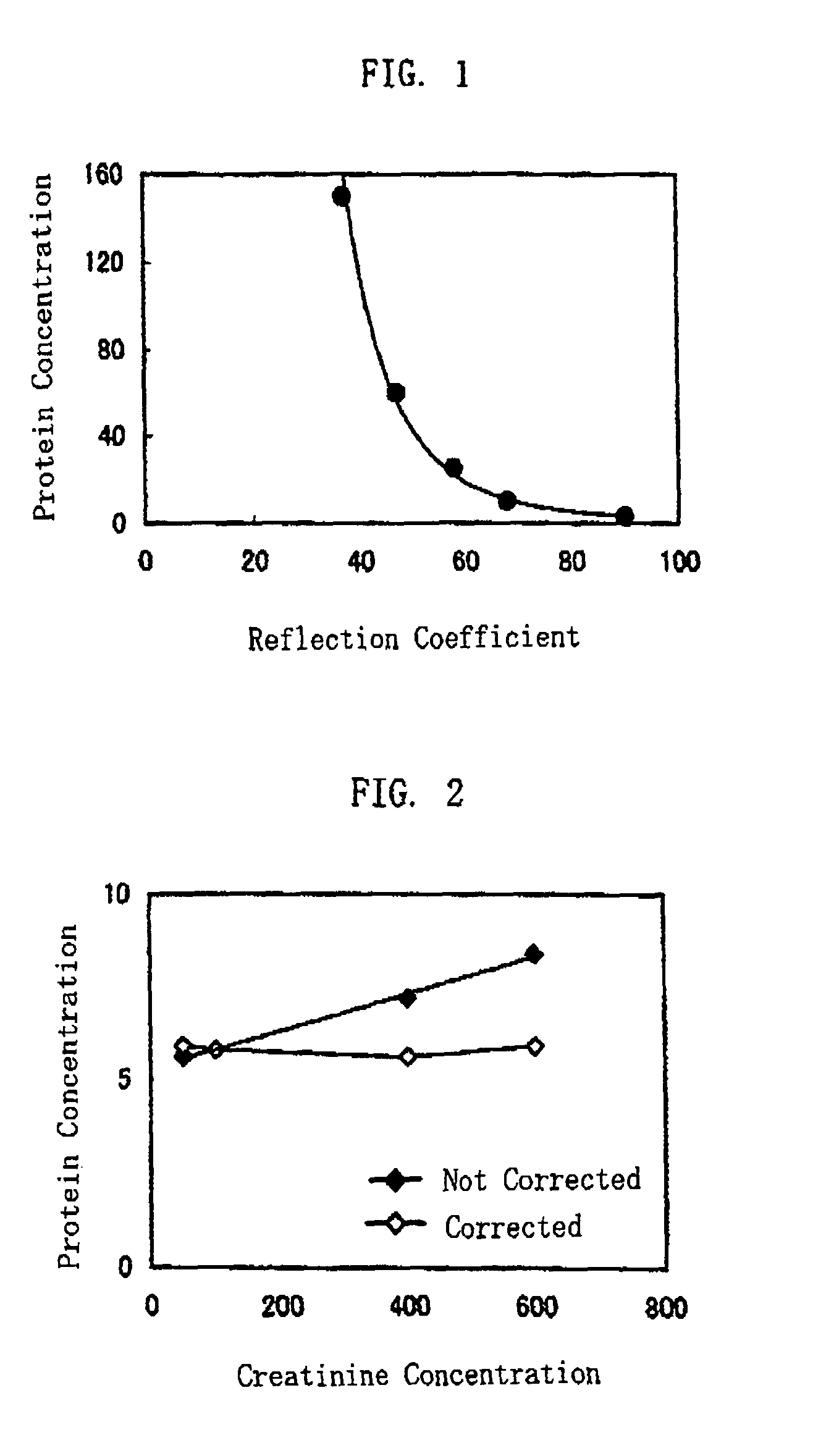
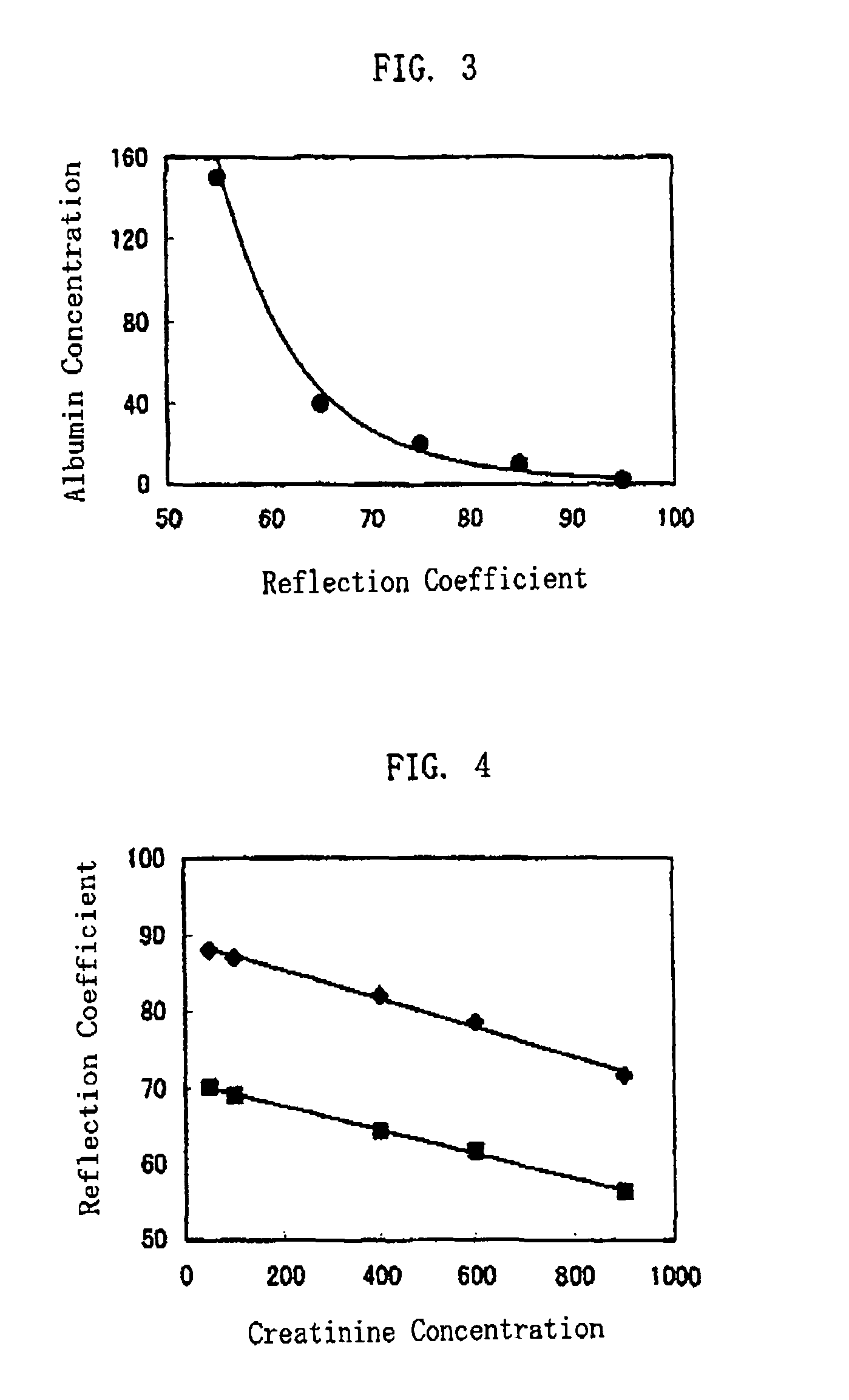
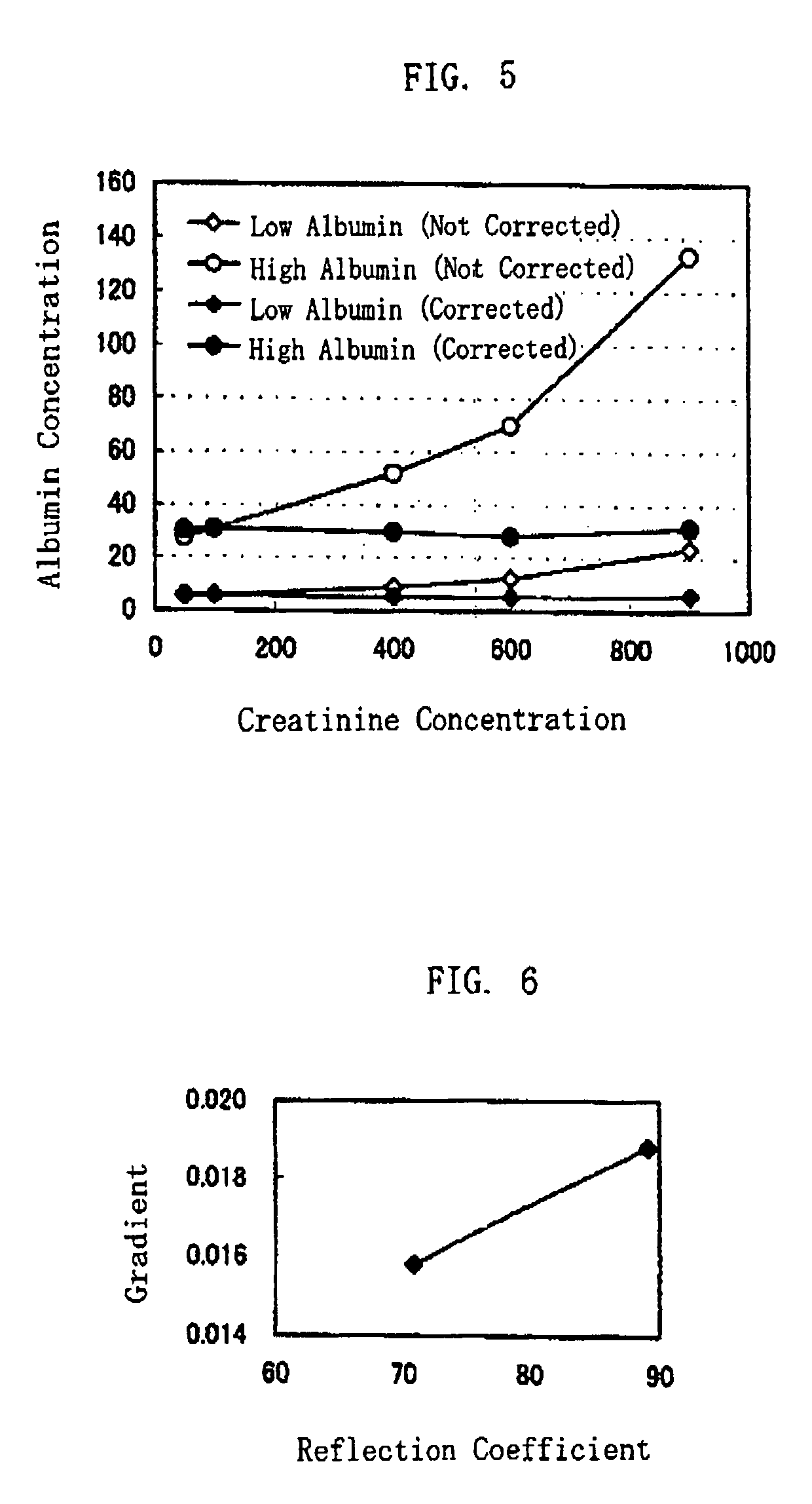
Method of Protein Measurement
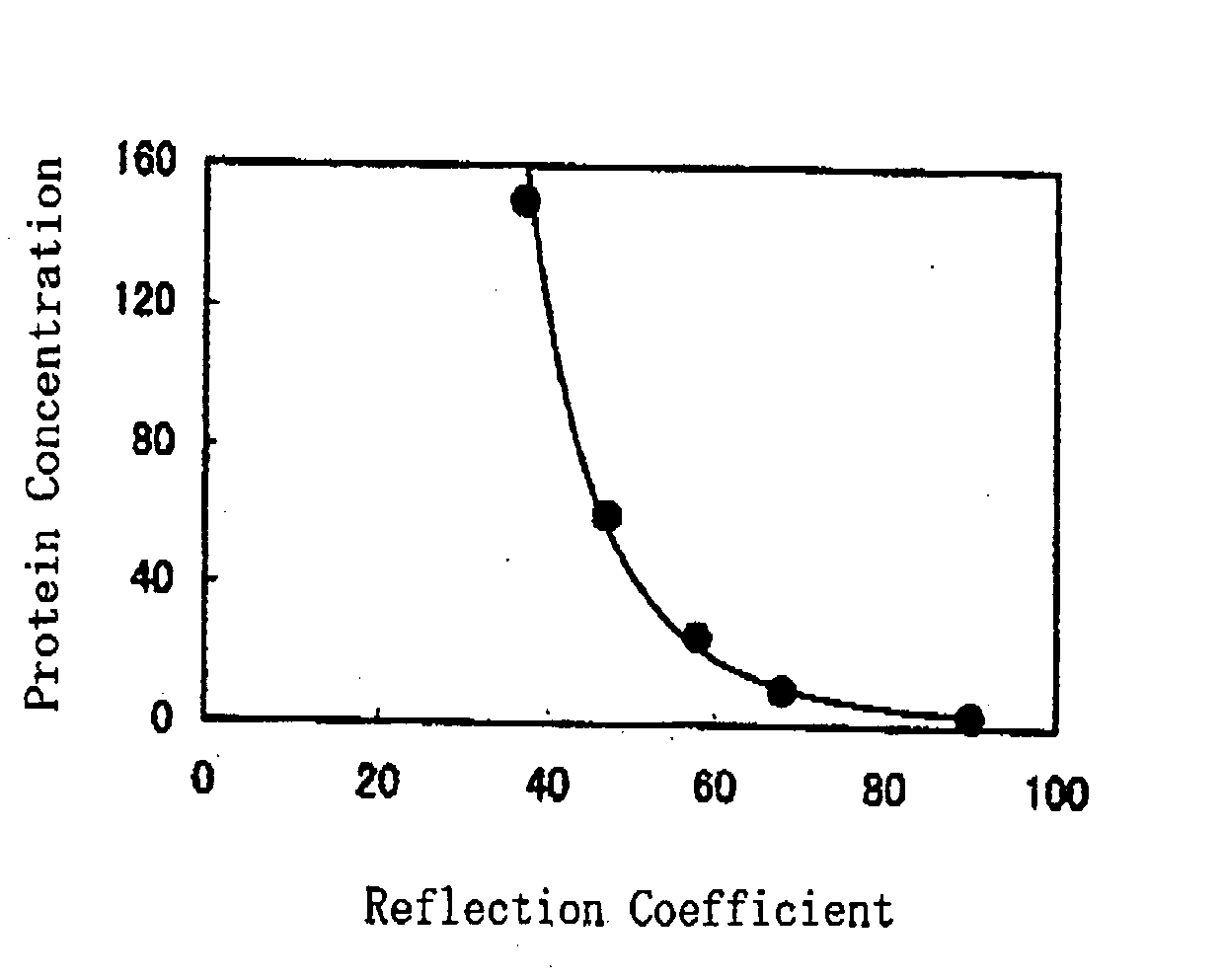
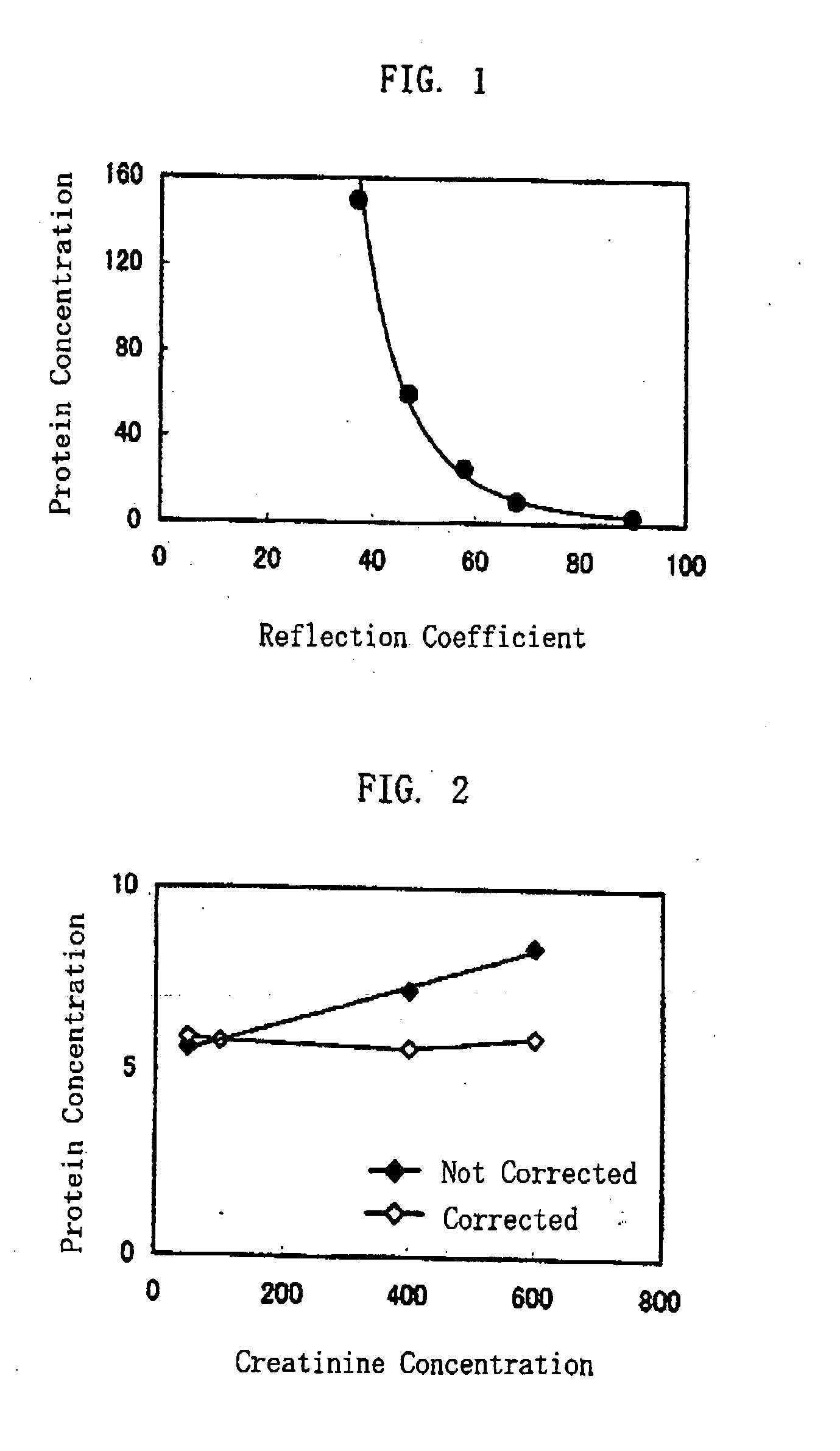
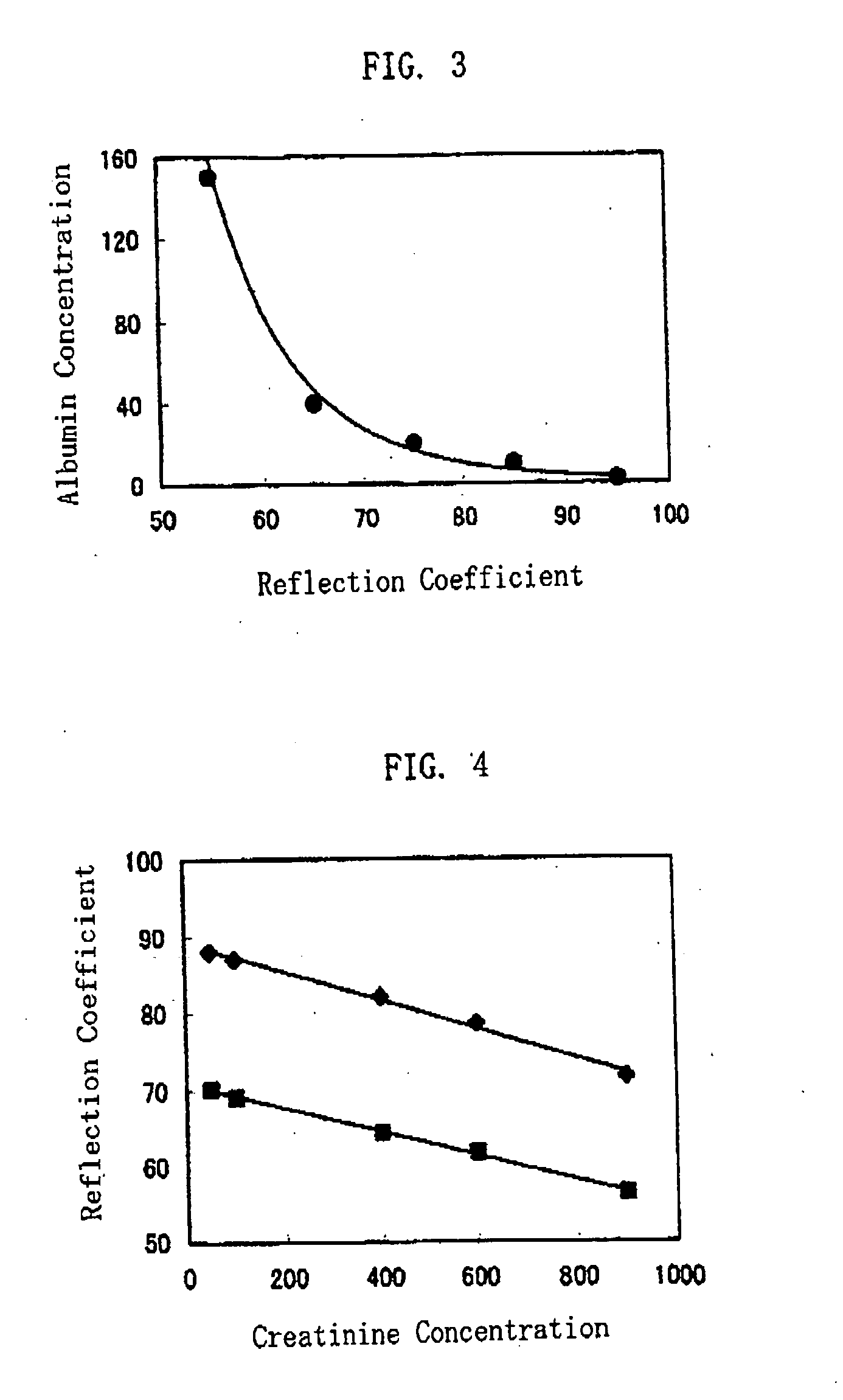
ActiveUS20080241850A1Accurate measurementEasy and cost-advantageous methodMaterial analysis by observing effect on chemical indicatorChemiluminescene/bioluminescenceCreatinine riseProtein concentration
The present invention relates to a technique of measuring a protein based on a degree of coloring in a liquid sample mixed with a protein measurement indicator. In the present invention, information reflecting creatinine concentration in the liquid sample is obtained, and then an influence quantity caused by creatinine to the protein concentration measurement is eliminated based on the information.
Owner:ARKRAY INC
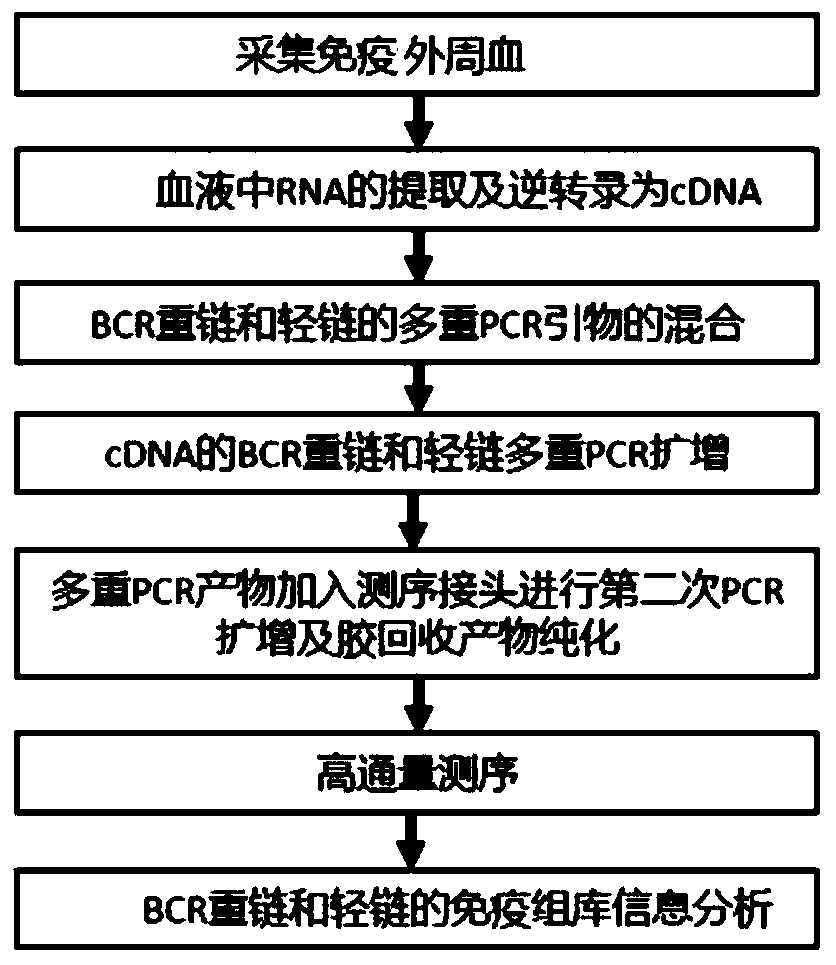
Immune repertoire method by detecting primers of BCR heavy and light chains in blood and application
ActiveCN111599411AUnderstand the lawEfficient synthesisMicrobiological testing/measurementSequence analysisSequence analysisStatistical analysis
The invention provides an immune repertoire information analysis method for detecting BCR heavy and light chains in blood. The method specifically comprises the steps of constructing a reference sequence, preprocessing sequencing data, merging Paired Reads, comparing data after Merge with the reference sequence, filtering a comparison result, and performing statistical analysis. Meanwhile, the invention further provides an immune repertoire method for detecting the blood BCR. The information analysis method is adopted in the method, sequencing analysis of a CDR3 region sequence is realized bydesigning a primer combination of the blood BCR heavy and light chains; the method is used for detecting the BCR immune repertoire heavy chain and light chain of rabbits for the first time. CDR3 sequence information related to antibody generation is studied through immune repertoire sequencing of blood B cells, the law of antibody generation is understood more deeply, and meanwhile, a new way forefficiently and rapidly synthesizing specific antibodies in vitro is explored by combining technologies such as gene cloning, cell transfection, protein determination and purification.
Owner:PROTEINT (TIANJIN) BIOTECHNOLOGY CO LTD
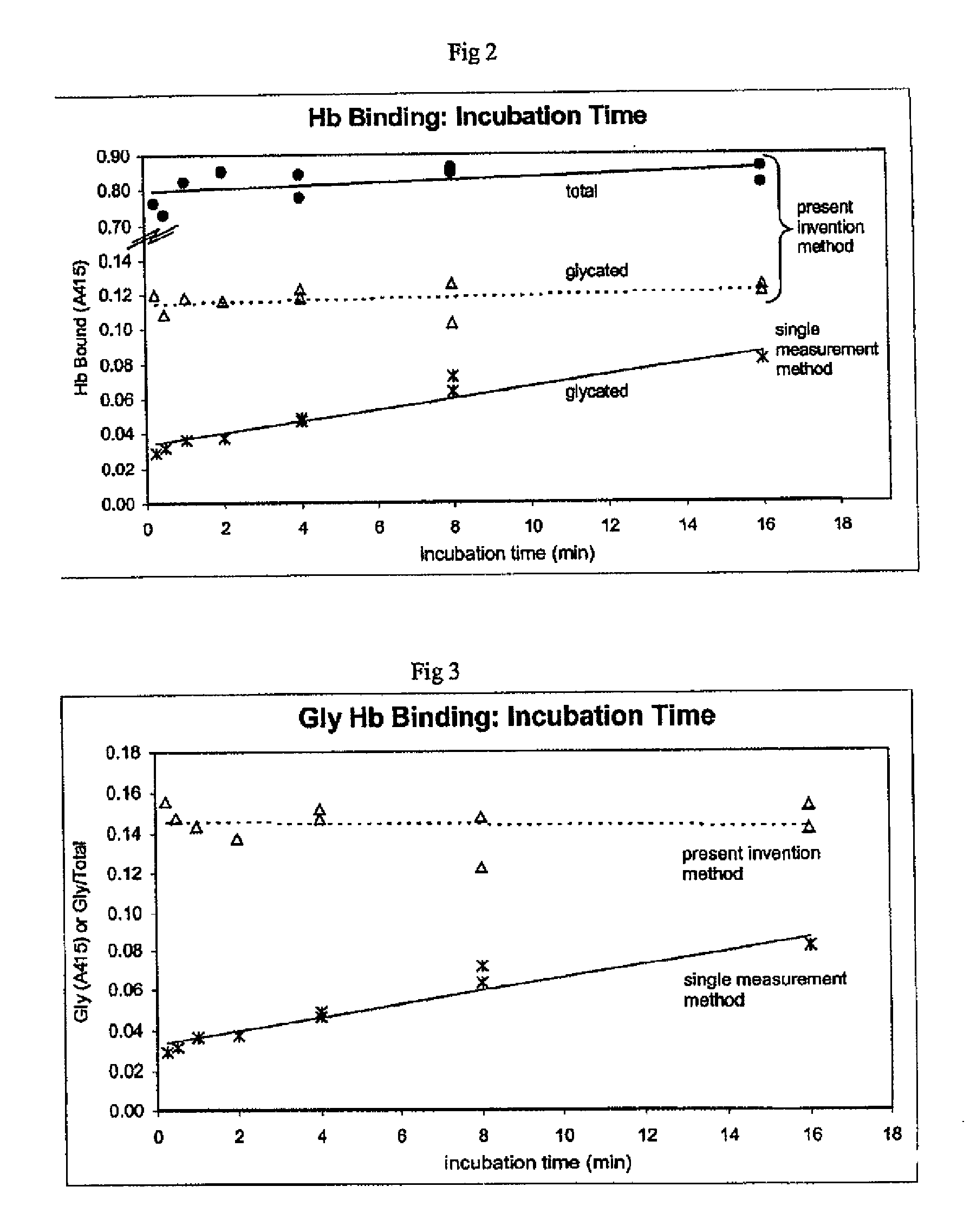
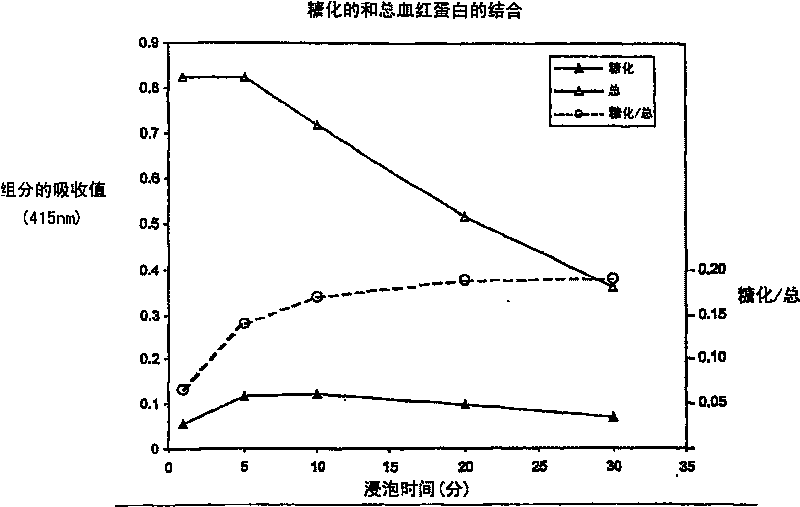
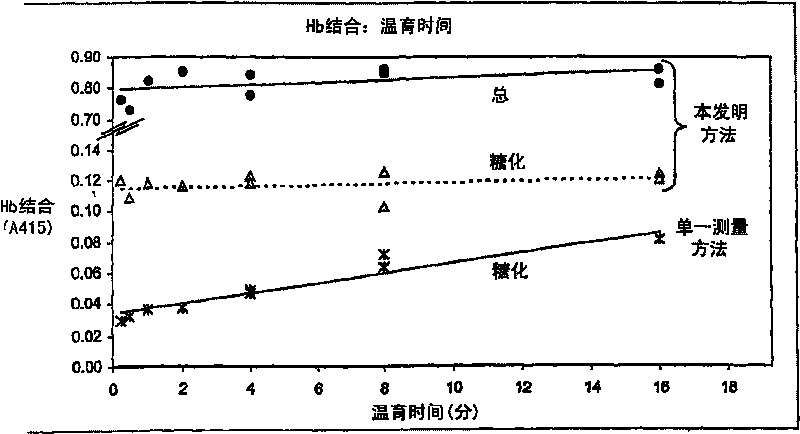
Determination of glycated protein
InactiveUS20070190658A1Immobilised enzymesBioreactor/fermenter combinationsSupport matrixGlycated protein
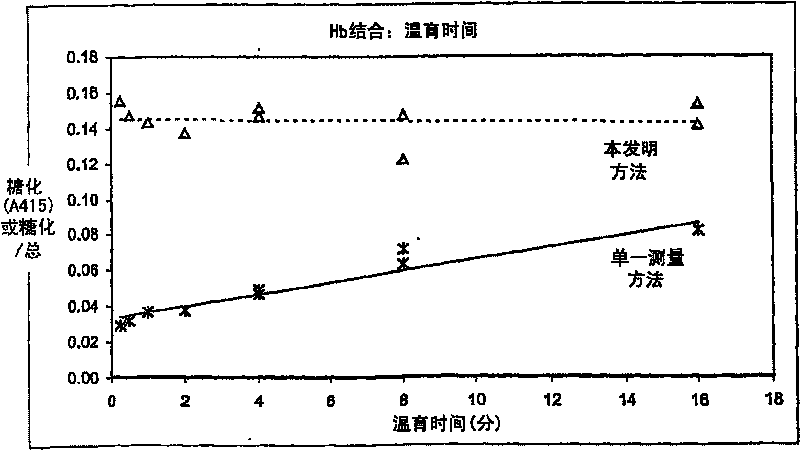
The present invention provides methods for quantitation of glycated protein in a biological sample using a solid support matrix by making a first bound protein measurement total bound protein under conditions where both glycated and non-glycated protein bind to the support in making a second bound protein measurement under conditions where glycated protein is bound to the support and non-glycated protein is not substantially bound. Diagnostic devices and kits comprising the methods of the present invention are also provided
Owner:BIOHERMES HK
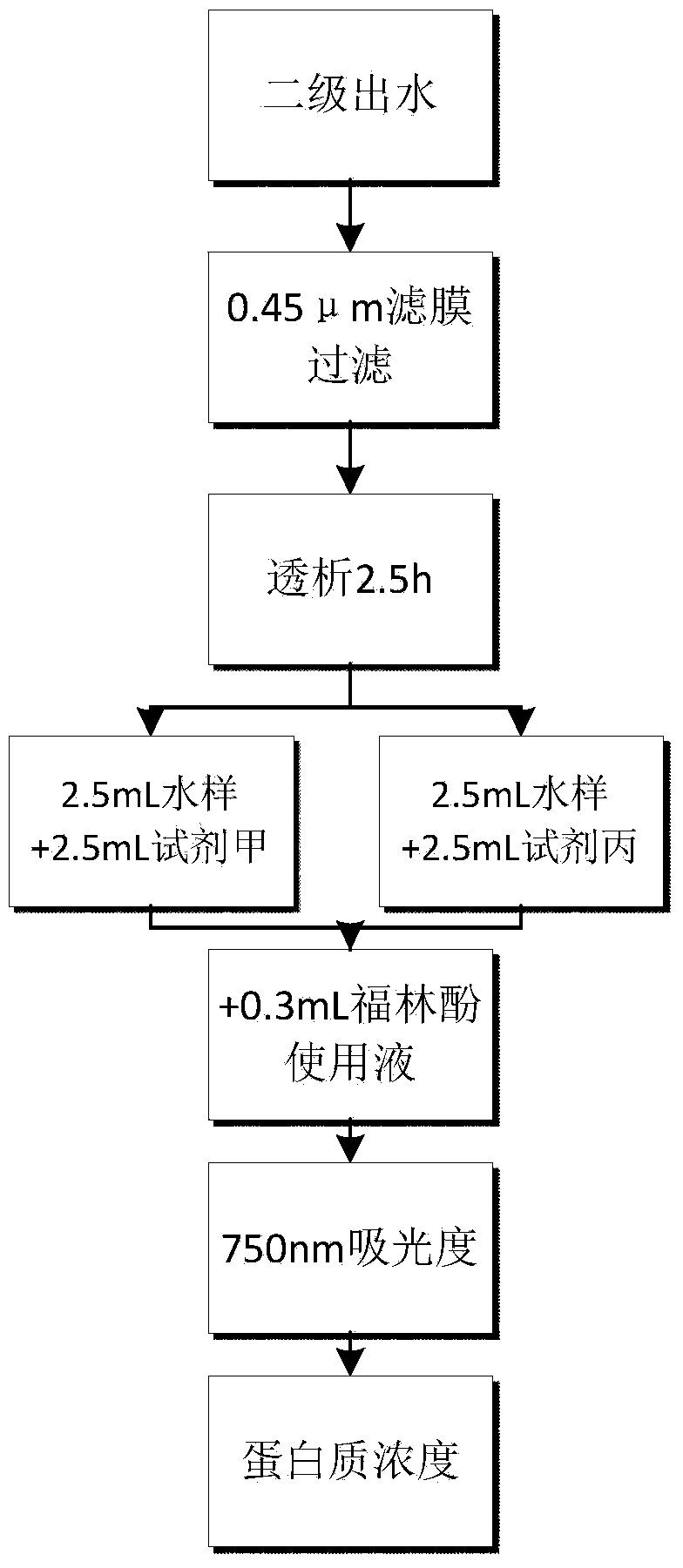
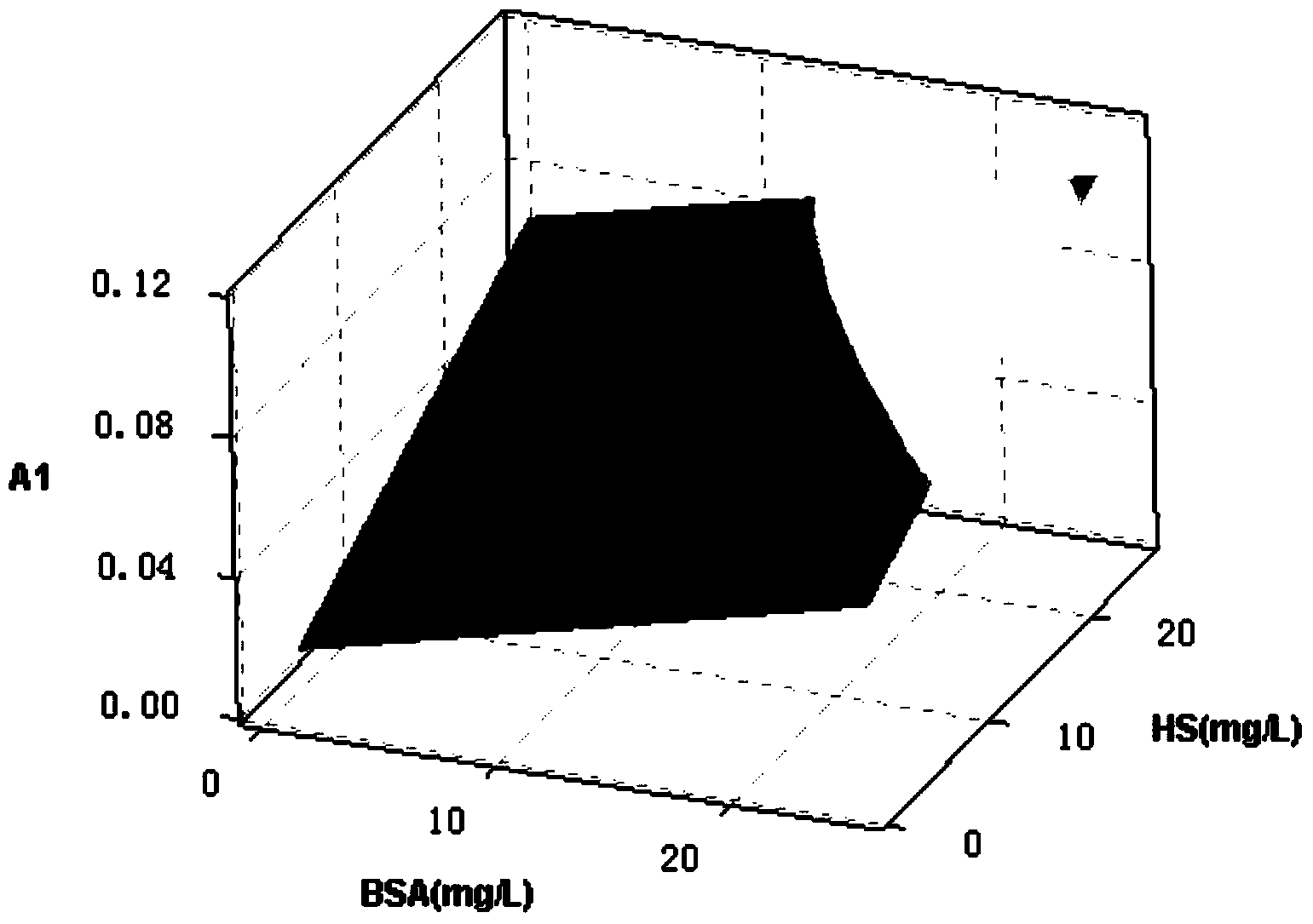
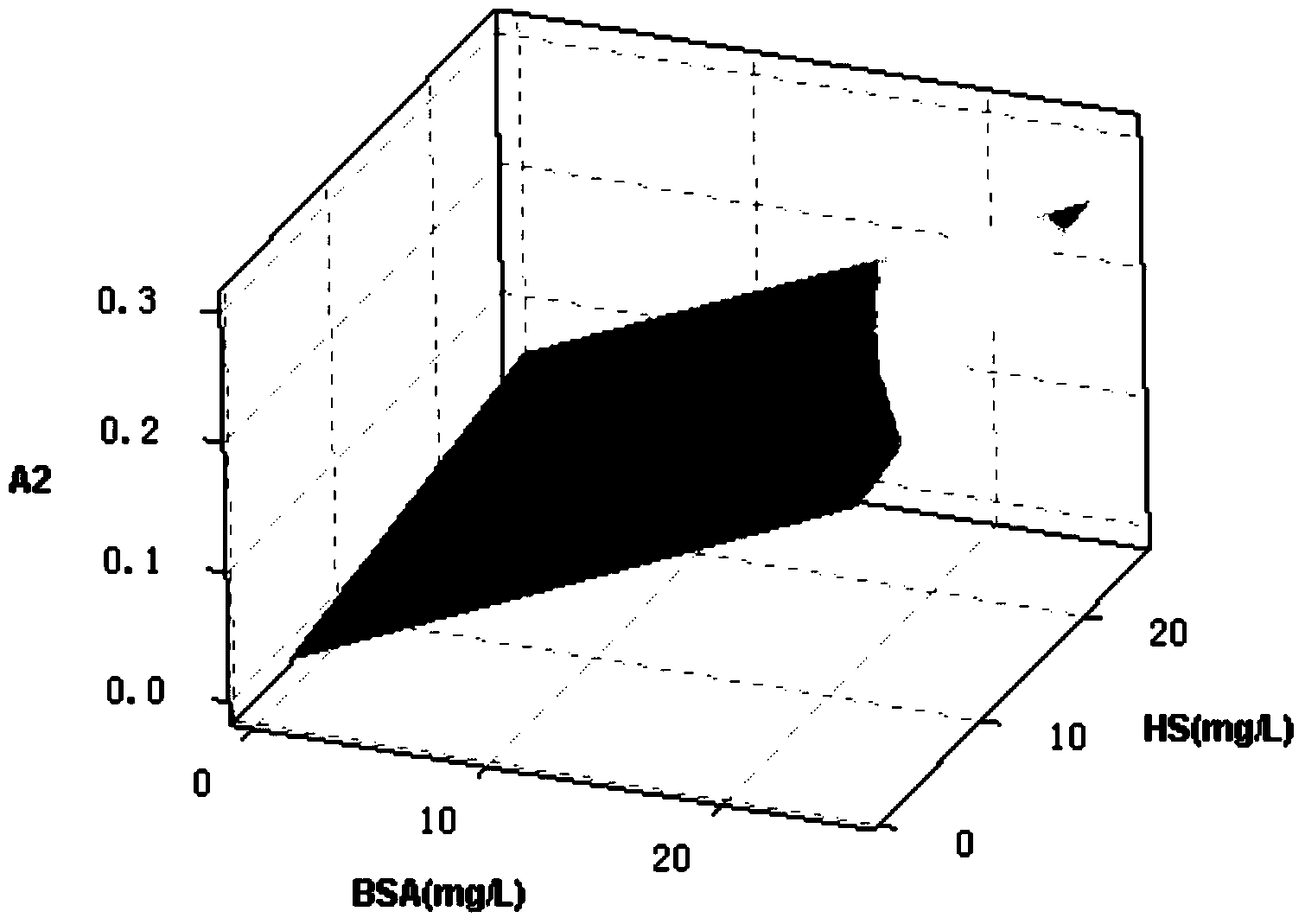
Method for determining low-concentration protein in secondary output water of sewage plant
ActiveCN104297182AReduce measurement errorSimple stepsColor/spectral properties measurementsSpecial data processing applicationsConcentration proteinMathematical model
The invention discloses a method for determining low-concentration protein in secondary output water of a sewage plant, belonging to the field of municipal sewage treatment. The method comprises the following steps: preparing a reagent, establishing a mathematical model, pretreating a sample, determining the concentration of the protein and the like. Based on a Lowry method and an improved Lowery method, a Minitab response surface method is used for analyzing the content of the protein so that the problems of low accuracy and low precision of the determination of the protein in real sewage are overcome; and the interferences on humic acid, carbohydrates, and ions including Ca<2+>, Mg<2+> and the like in the secondary output water are reduced. The method for determining the low-concentration protein in the secondary output water of the sewage plant has the advantages of high accuracy, simplicity, easiness for operation and the like, is suitable for determining the low-concentration protein in the secondary output water of the sewage plant, and can be used for synchronously determining the concentration of the humic acid in the secondary output water, so that the applicable range is wide.
Owner:NANJING UNIV
C-reactive protein determination reagent and preparation method thereof
InactiveCN110031636AGood effectAnalytical sensitivityBiological material analysisBiological testingMicrospherePhosphate
The invention provides a C-reactive protein determination reagent. The C-reactive protein determination reagent comprises a C-reactive protein detection reagent R1, a C-reactive protein detection reagent R2 and a C-reactive protein calibrator; buffer solution is prepared from 4- hydroxyethylpiperazine ethane sulfonic acid-sodium hydroxide buffer solution, trihydroxymethyl aminomethane-hydrochloricacid buffer solution, 3-(N-morpholinyl)-2-hydroxyl propanesulfonic acid-sodium hydroxide buffer solution and phosphate buffer solution according to mass ratio of 1:1:1:1; the C-reactive protein immune gel emulsion microspheres are polystyrene microspheres, preservative is one or two of proclin 300, gentamicin and chloramphenicol. The buffer solution used for the reagent provided by the inventionis prepared from the 4- hydroxyethylpiperazine ethane sulfonic acid-sodium hydroxide buffer solution, the trihydroxymethyl aminomethane-hydrochloric acid buffer solution, the 3-(N-morpholinyl)-2-hydroxyl propanesulfonic acid-sodium hydroxide buffer solution and the phosphate buffer solution according to mass ratio of 1:1:1:1, and the reagent is remarkable effects; and the reagent has the advantages of high accuracy, high measurement precision, high analysis sensitivity, and so on.
Owner:贵州盛世康生物科技有限公司
Method for confirming tumour differentiation grade
InactiveCN1764727APredicted grade of differentiationMicrobiological testing/measurementDrug compositionsHuman tumorOncology
The present invention relates to a method of defining the differentiation grade of tumor by selecting genes and / or proteins whose expression level correlates with each differentiation grade of tumor, measuring the expression of the genes and / or proteins of human tumor tissues in each differentiation grade. The present invention also relates to the use of these genes and / or proteins for diagnosing the differentiation grade of tumor and for screening anti-cancer agents for tumor treatment.
Owner:F HOFFMANN LA ROCHE & CO AG
Specific protein analyzer, determination method and computer readable storage medium
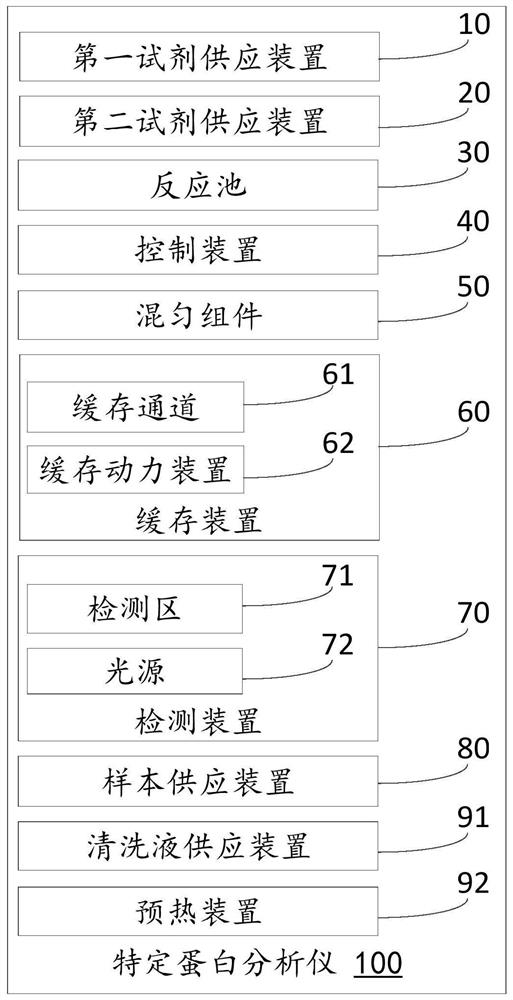
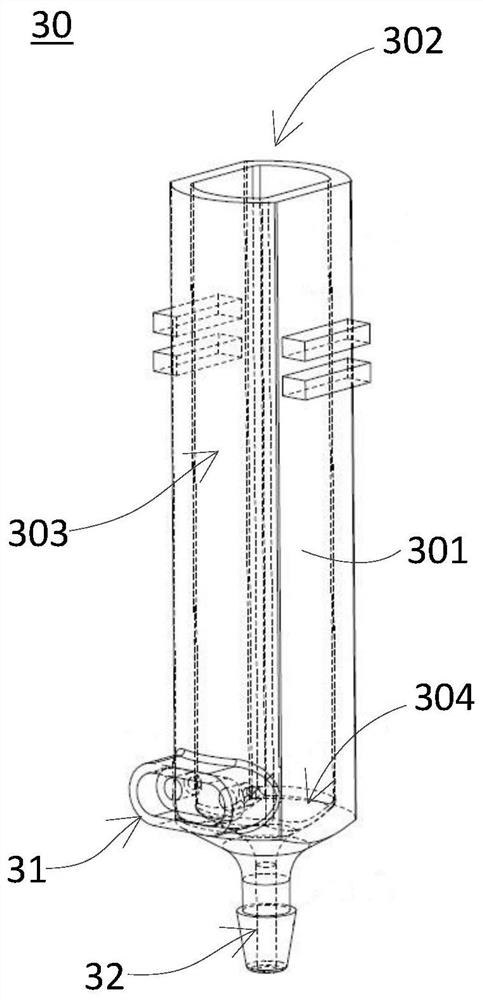
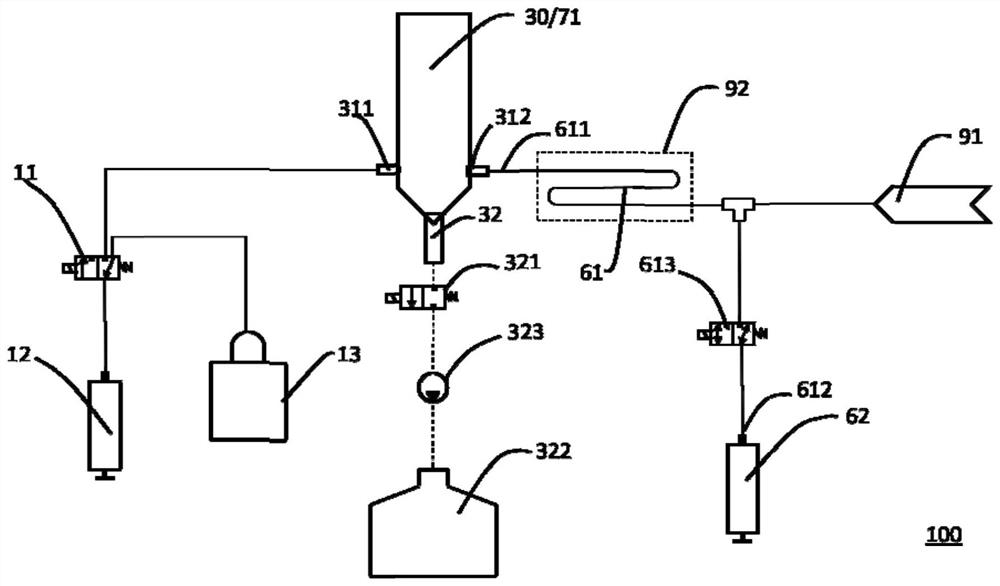
PendingCN113884688AReduce air bubble contentImprove detection accuracyScattering properties measurementsTransmissivity measurementsFluid phaseBlood specimen
The invention discloses a specific protein analyzer. The specific protein analyzer comprises a sample supply device, a first reagent supply device, a second reagent supply device, a reaction tank, a uniform mixing assembly, a cache assembly, a detection device and a control device, wherein the sample supply device, the first reagent supply device and the second reagent supply device are respectively used for supplying a blood sample to be detected, a first reagent and a second reagent into the reaction tank; the uniform mixing assembly is used for uniformly mixing the mixed sample liquid, then the control device is used for controlling the cache assembly to suck out the mixed sample liquid in the middle of the reaction tank for caching, and specific protein content detection of the detection device is received. The mixed sample liquid in the middle of the reaction tank is relatively uniform and contains a small number of bubbles so that a more accurate detection effect can be obtained. The invention further provides a specific protein determination method and a computer readable storage medium for implementing the method.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
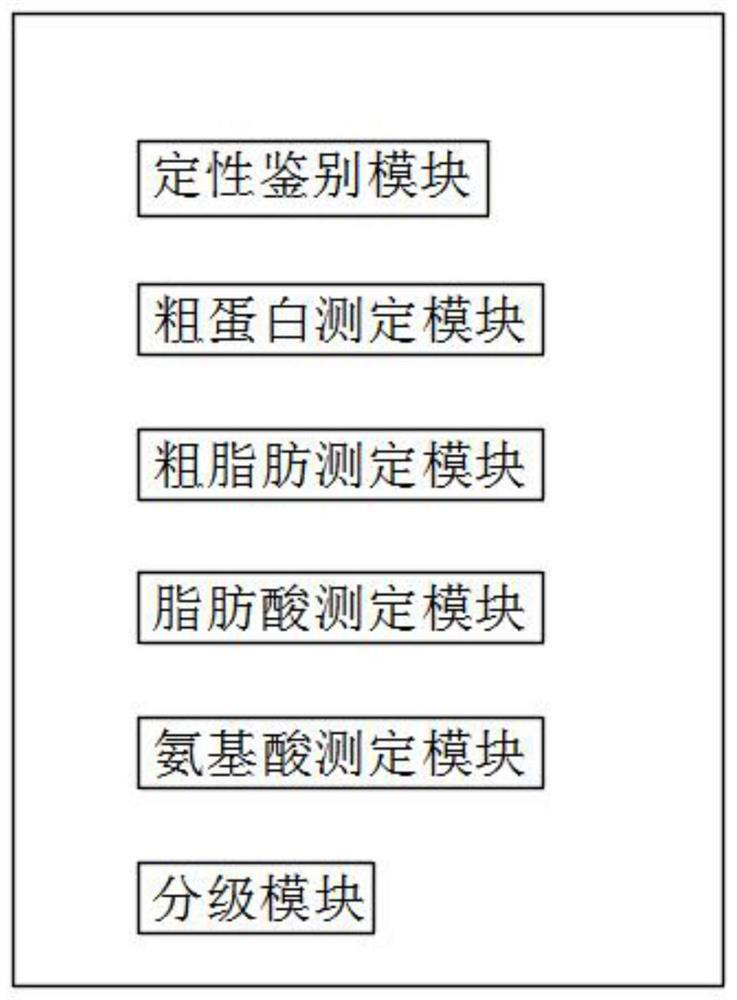
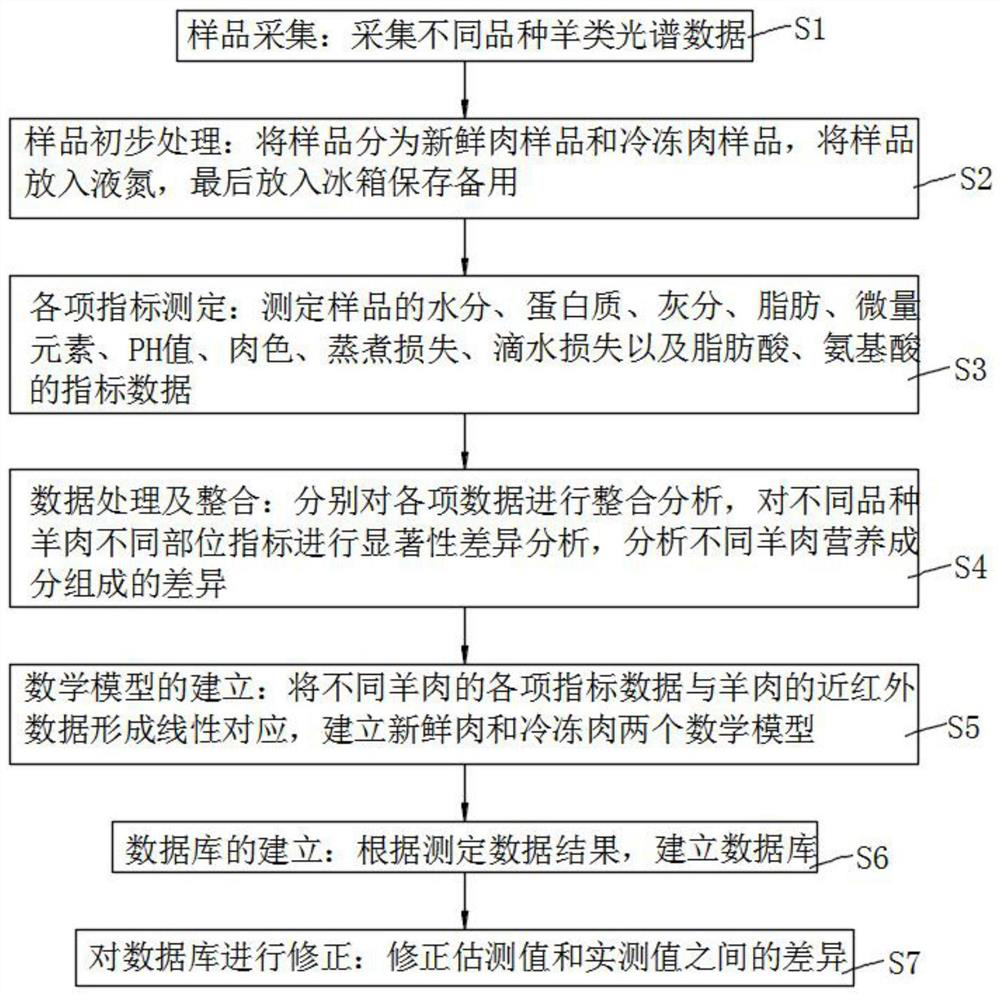
Database for rapidly detecting mutton quality based on near infrared and establishment method thereof
InactiveCN112595694AFill in the gaps in quantitative indicatorsImprove detection accuracyMaterial analysis by optical meansBiotechnologyNutritional Indices
The invention provides a database for rapidly detecting mutton quality based on near infrared and an establishment method thereof, and relates to the technical field of mutton quality detection. The database comprises a qualitative identification module, a crude protein determination module, a crude fat determination module, a fatty acid determination module, an amino acid determination module anda grading module. According to the invention, by establishing a mutton database containing crude protein, crude fat, amino acid, fatty acid and other nutritional indexes, meat color, brightness, PH and other physical indexes, and corresponding spectral information of mutton, the operation is simple, the cost is low, the purposes of quantitative analysis of nutritional ingredients and quality grading can be achieved through simple spectral scanning, and meanwhile, a reliable detection model is also provided, the detection accuracy of crude protein, crude fat, amino acid and fatty acid of nutritional indexes is extremely high, the blank of quantitative indexes of mutton grading is filled, and the grading accuracy is 95% or above.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
Method for evaluating influences of biological protein on apolygus lucorum
InactiveCN103609529AEfficient determinationAccurate measurementAnimal feeding stuffArthropod mouthpartsFodder
The invention provides a method for evaluating influences of biological protein on apolygus lucorum. The method includes the steps of firstly, diluting biological protein to be measured to obtain biological protein with different concentrations, mixing the biological protein with apolygus lucorum artificial fodders to obtain biological protein measurement fodders, and taking fodders where biological protein is not added as a contrast; secondly, wrapping the measurement fodders and the contrast fodders with extended sealing films respectively to obtain capsules; thirdly, adhering the capsules to the side wall of a raising box without cover; fourthly, inoculating nymphs of apolygus lucorum into the raising box, sealing the opening of the raising box through gauze, and conducting cultivation; fifthly, recording the death condition of apolygus lucorum or the weight changes of apolygus lucorum from the day 0, and thereby evaluating the influences of biological protein on apolygus lucorum. The method is wide in application range, agents with stomach toxicity and systemic action and chemical substances dissolved in the artificial fodders can be effectively measured, the problem that piercing-sucking mouthpart insects including apolygus lucorum can hardly make direct contact with biological protein with different concentrations is solved, the influences of biological protein on apolygus lucorum can be accurately measured, and the method has high practicability.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Method of protein measurement
ActiveUS8460877B2Accurate methodExclude influenceMaterial analysis by observing effect on chemical indicatorChemiluminescene/bioluminescenceCreatinine riseProtein measurement
The present invention relates to a technique of measuring a protein based on a degree of coloring in a liquid sample mixed with a protein measurement indicator. In the present invention, information reflecting creatinine concentration in the liquid sample is obtained, and then an influence quantity caused by creatinine to the protein concentration measurement is eliminated based on the information.
Owner:ARKRAY INC
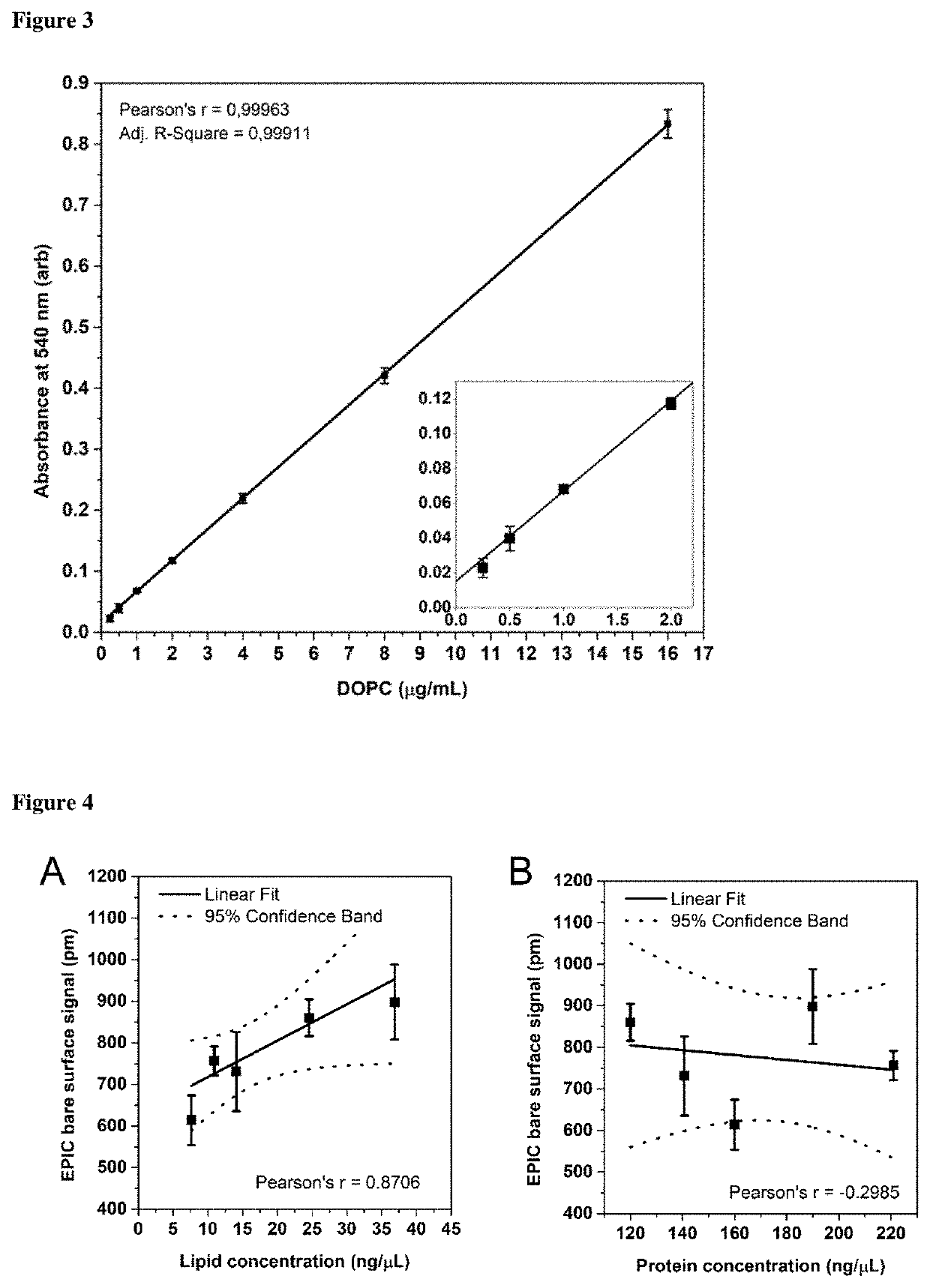
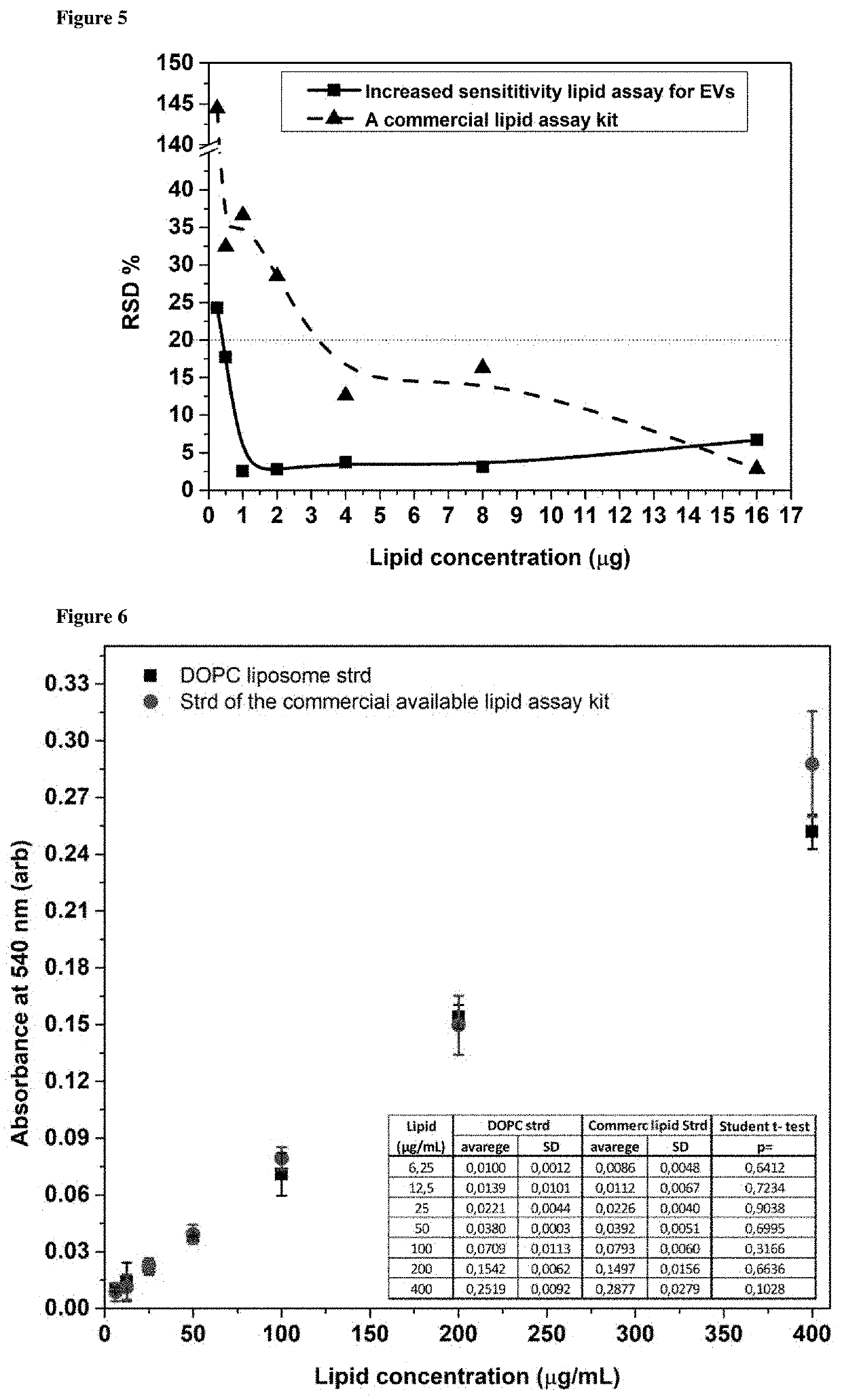
Method for determining the lipid content of extracellular vesicles
PendingUS20210293834A1Material analysis by observing effect on chemical indicatorOmicsDiseaseCholesterol
The field of extracellular vesicles (EVs) currently attracts substantial attention in biomedicine due to the proposed role of EVs in various biological processes and their potential of serving as biomarkers for diseases. However, the availability of approaches for reliable and reproducible standardised quantification of EVs is limited. Therefore, current interest in EV research urges reliable tools of standardization and accurate enumeration of EVs, preferably on the basis of lipid quantification. By definition, EVs are surrounded by phospholipid bilayers, therefore, lipids (such as phospholipids and cholesterol) are essential components of all EVs. The method of the invention avoids the overestimation of EV concentration based on the protein measurement, as it focuses on the defining component of EVs, the lipid bilayer. Our method can be used virtually in any standard laboratories where a fume hood, a thermoblock, and a spectrophotometer are available. The application does not require expensive equipment, therefore it can be an easy, reliable and quick method for quantification of EVs and standardisation of EV experiments.
Owner:RES CENT FOR NATURAL SCI
Method and devices for quantitation of glycated protein
InactiveCN1489693BEasy to useBiological material analysisColor/spectral properties measurementsSupport matrixProtein insertion
The present invention provides methods for quantitation of glycated in a biological sample using a solid support matrix by making a first bound protein measurement total bound protein under conditionswhere both glycated and non-glycated protein bind to the support and making a second bound protein measurement under conditions where glycated protein is bound to the support and non-glycated proteinis not substantially bound. Diagnostic devices and kits comprising the methods of the present invention are also provided.
Owner:无锡博慧斯生物医药科技有限公司
Quantitative detection method of goat pox virus
ActiveCN114544815AEasy to operateAddressing the inability to accurately quantify intact virus contentComponent separationAgainst vector-borne diseasesTGE VACCINEBiomedical engineering
The invention discloses a quantitative detection method of a goat pox virus. The method comprises the following steps: firstly, identifying a target peak by using a virus specificity detection method; determining the concentration of the standard substance by using a protein determinator; carrying out series dilution on the standard substance, detecting the absorption peak of the standard substance at 280nm on a liquid chromatograph, and establishing a linear regression equation of concentration and peak area; and calculating the content of the goat pox virus in the sample to be detected according to the linear regression equation and the peak area of the sample to be detected. The method disclosed by the invention can be used for determining the content of the goat pox virus in a goat pox virus suspension culture solution, a purified solution and a goat pox inactivated vaccine, has the advantages of rapidness, accuracy, stability and good repeatability, can guide the production of vaccines, and plays an important role in improving the quality of the vaccines.
Owner:CHINA ANIMAL HUSBANDRY IND
Method for confirming tumour differentiation grade
InactiveCN1764727BPredicted grade of differentiationMicrobiological testing/measurementDrug compositionsHuman tumorOncology
The present invention relates to a method of defining the differentiation grade of tumor by selecting genes and / or proteins whose expression level correlates with each differentiation grade of tumor, measuring the expression of the genes and / or proteins of human tumor tissues in each differentiation grade. The present invention also relates to the use of these genes and / or proteins for diagnosingthe differentiation grade of tumor and for screening anti-cancer agents for tumor treatment.
Owner:F HOFFMANN LA ROCHE & CO AG
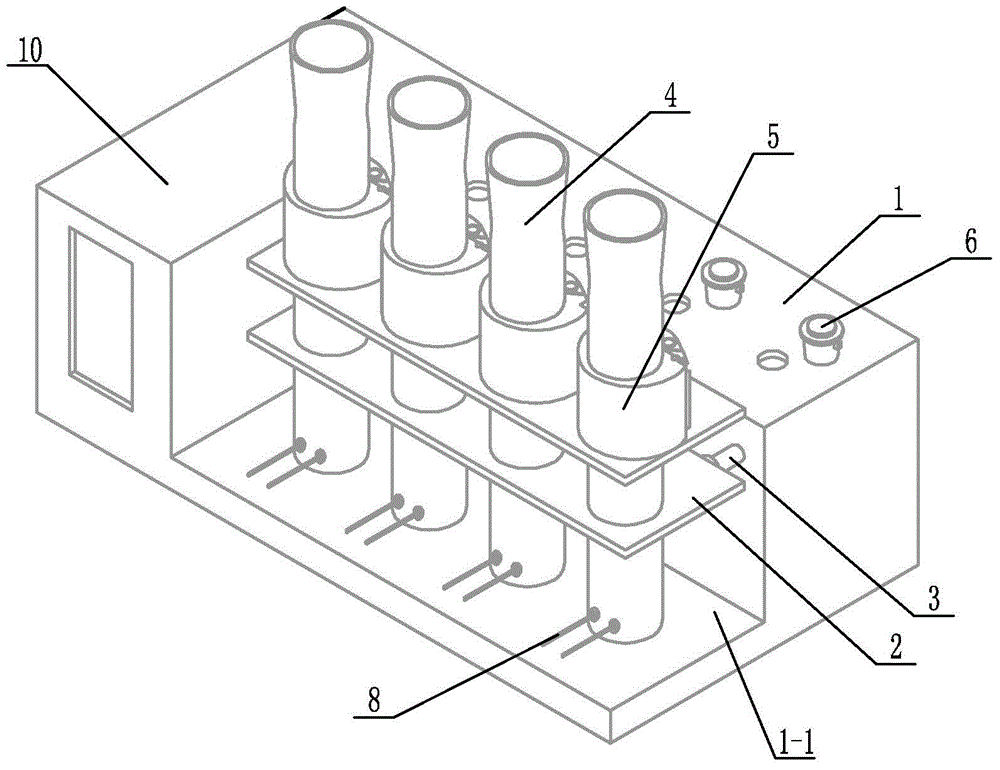
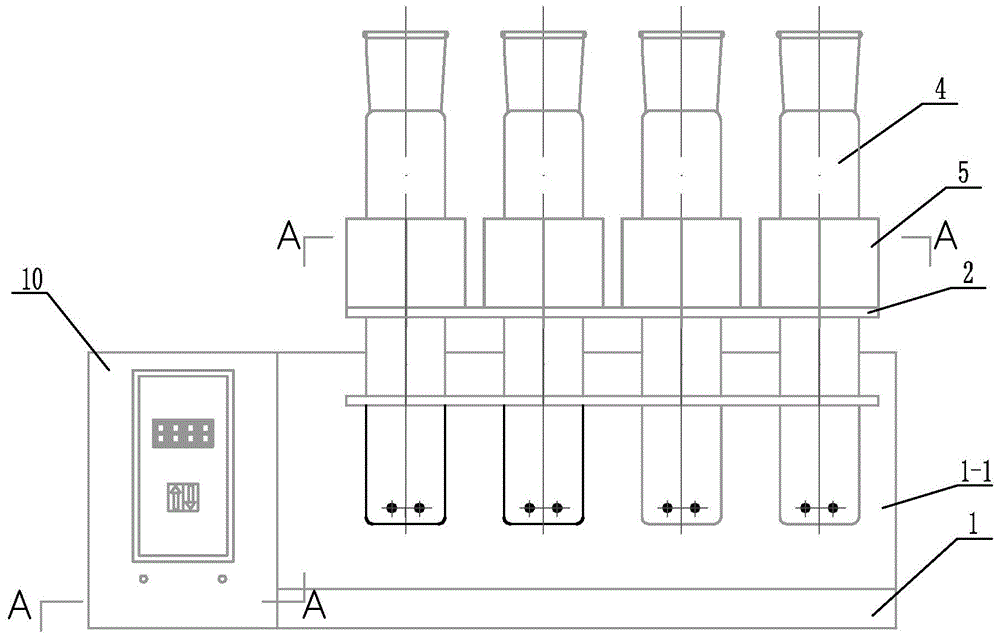
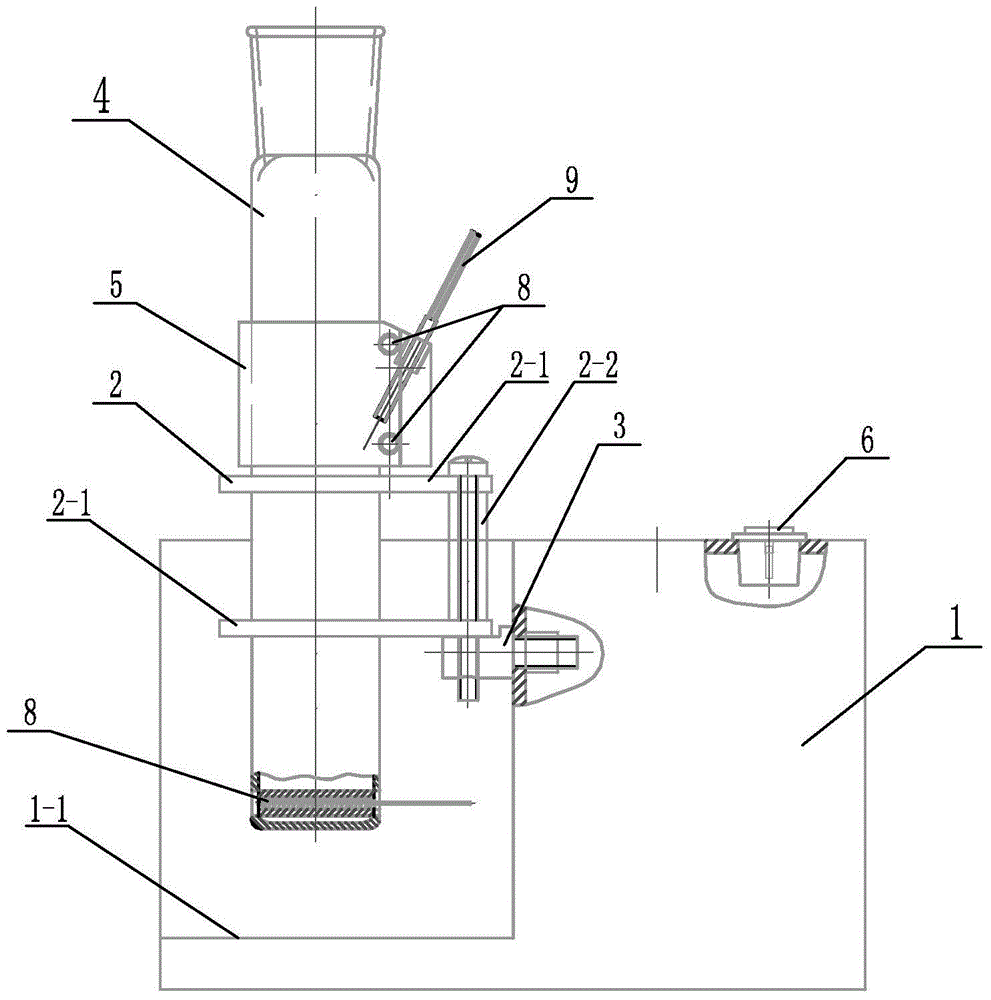
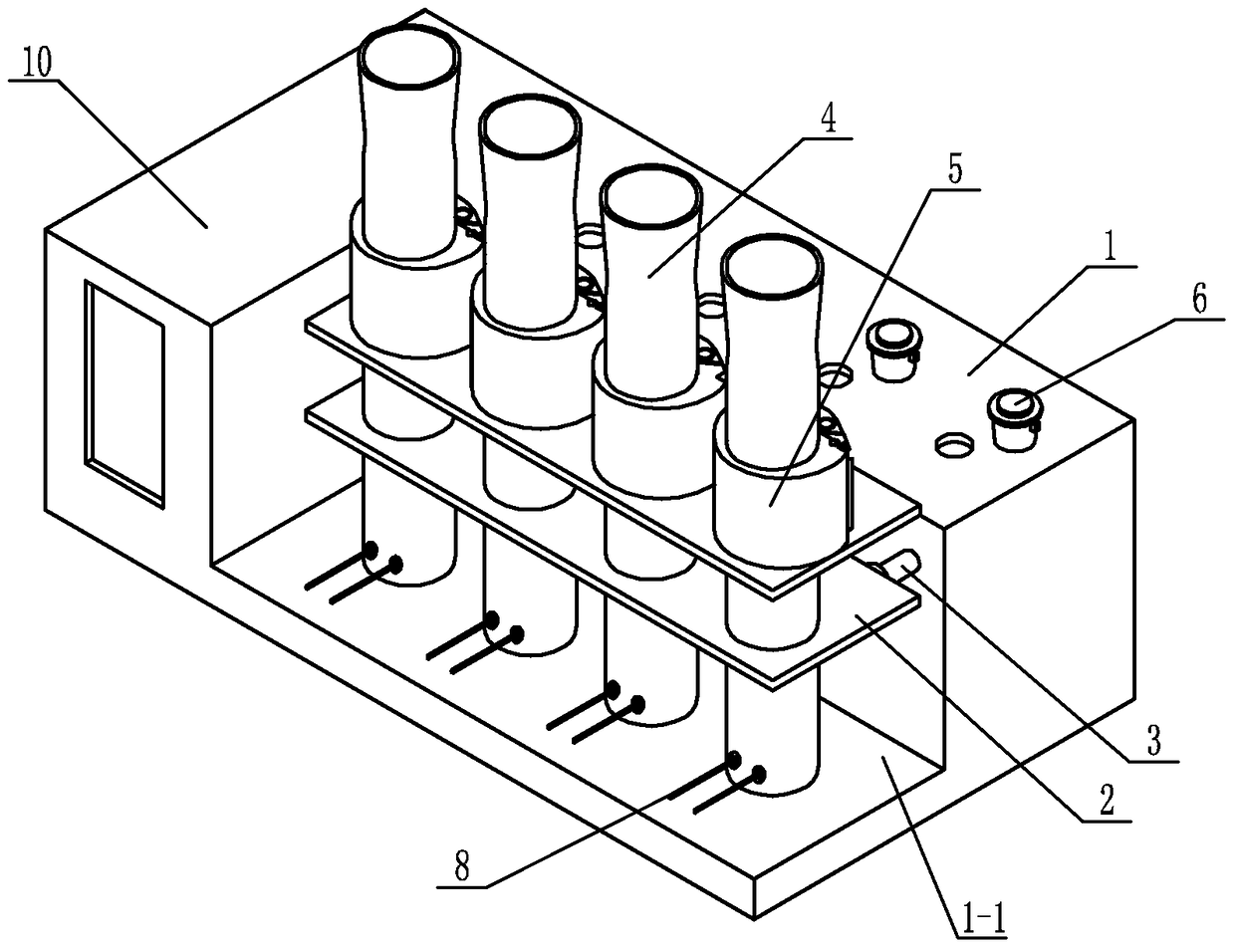
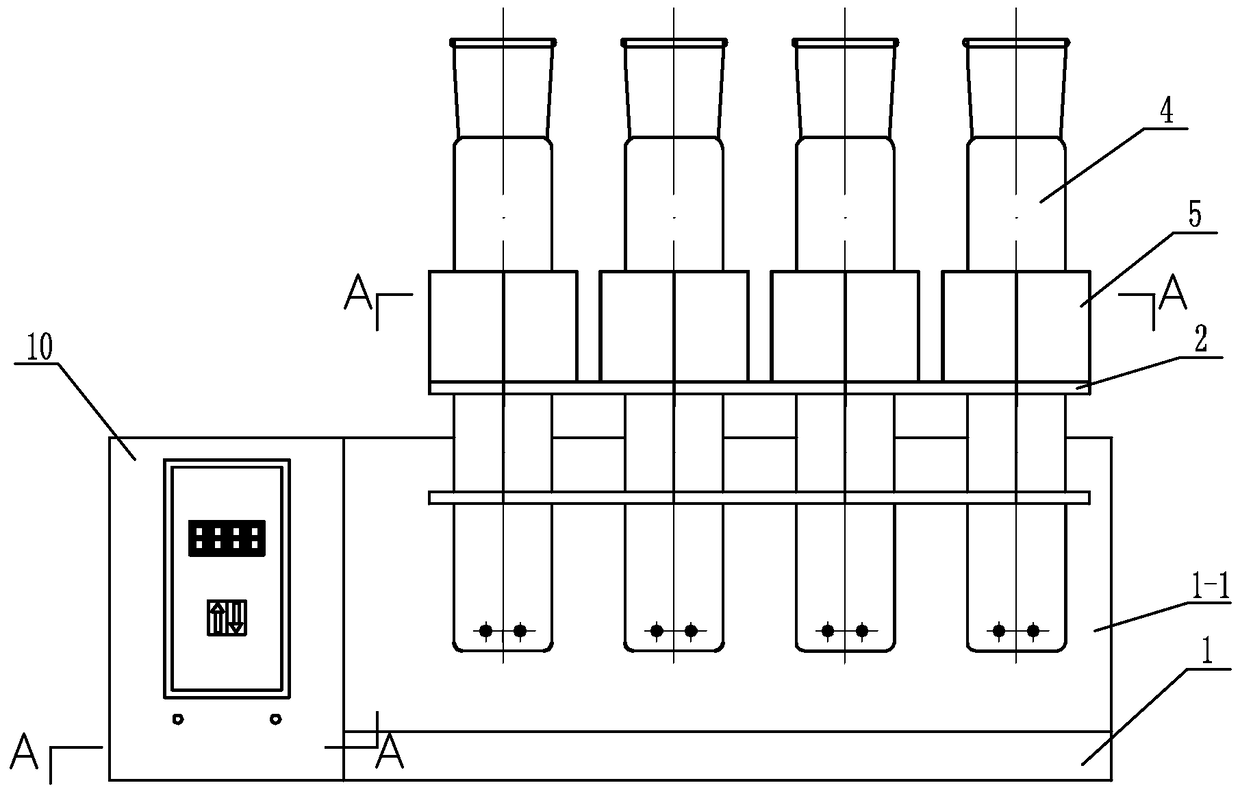
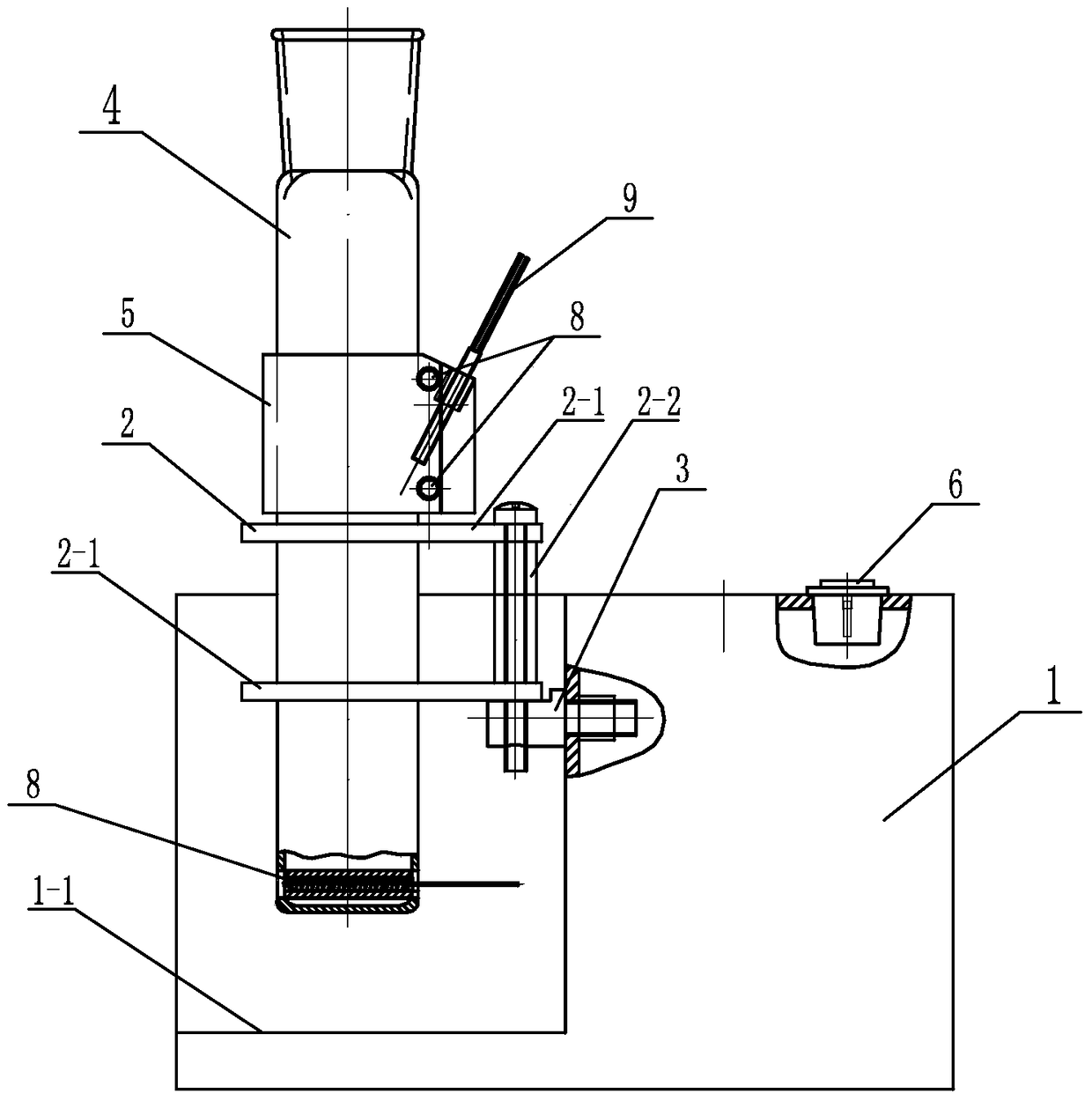
Digestion device for protein measurement
ActiveCN104977206AHeating up fastRelieve pressurePreparing sample for investigationTemperature controlEngineering
Disclosed is a digestion device for protein measurement, which relates to a digestion device. A right-angle container is formed in the front end face of a chassis; an electric appliance control box is arranged at one side of the chassis and at an end part of the right-angle container; a plurality of transverse foundation pillars are arranged in the right-angle container in parallel and are connected to the chassis; a mounting base is arranged on and connected to the transverse foundation pillars; a plurality of internal heating digestion pipes are vertically arranged in the mounting base in parallel; a lower part of each internal heating digestion pipe is provided with two transverse holes internal heating digestion pipe perpendicular to axes of the internal heating digestion pipes; each transverse hole is internally provided with a through-tube; each through-tube is internally provided with a U-shaped heating wire; each internal heating digestion pipe is corresponding to a switch and a temperature control base; the temperature control bases are fixedly sleeved in the internal heating digestion pipes; the side wall of each temperature control base is equipped with a temperature sensor and two U-shaped heating wires, wherein the temperature sensor and the U-shaped heating wires are connected to the electric appliance control box via conductive wires; and a plurality of switches are installed on the upper end face of the chassis, and are connected to the control box through conductive wires. The digestion device is used for measuring protein in various biological and plant samples.
Owner:QIQIHAR UNIVERSITY
Milk product true protein determinator and determination method thereof
InactiveCN105067675ARapid determinationAccurate measurementPreparing sample for investigationMaterial analysis by electric/magnetic meansProtein detectionProtein nitrogen
The invention discloses a milk product true protein determinator and a determination method thereof, and belongs to the technical field of milk product true protein detection. The milk product true protein determinator comprises a potentiometer, a dissolved oxygen electrode, a test cup body, a magnetic stirrer and a current detection device; the bottom of the test cup body is communicated with and fixed on the magnetic stirrer, a stirring rod located on the magnetic stirrer is arranged in an inner cavity of the test cup body, the dissolved oxygen electrode is connected with the potentiometer through a wire, the bottom of the dissolved oxygen electrode stretches into the inner cavity of the test cup body, the current detection device is used for detecting currents of a solution in the inner cavity of the test cup body, and the potentiometer, the dissolved oxygen electrode, magnetic stirrer and the current detection device are controlled by a control system to work. According to the milk product true protein determinator and the determination method thereof, the content of protein in milk products is quickly determined by means of a current method, the content of the protein in the sample is analyzed by determining the current decrease value through a dissolved oxygen meter, and therefore the detection errors caused by existence of non-protein nitrogen are reduced.
Owner:SICHUAN KAFU DETECTION TECH CO LTD
Multiplexing transcription factor reporter protein assay process and system
A protein reporter system comprising at least one reporter including a response element responsive to the binding of a transcription factor, a secreted enzyme backbone and a recognition region for specific binding of an antibody. Multiplexed assays for binding, assaying and quantifying the activity of transcription factors are also described, in which the assays use protein reporters in sets, libraries or other groupings, as necessary to achieve desired quantification.
Owner:ATTAGENE
A protein determination and digestion instrument
ActiveCN104977206BHeating up fastRelieve pressureHeating or cooling apparatusTemperature controlEngineering
A protein determination and digestion instrument relates to a digestion instrument. There is a right-angle slot on the front end of the chassis, and the end of the side of the chassis located in the right-angle slot is the electrical control box. Several horizontal base columns are arranged in parallel in the right-angle slot and connected with the chassis. The base column is connected, and several internal heat digestion tubes are vertically and parallelly arranged in the installation seat. The lower part of each internal heat digestion tube is provided with two horizontal holes perpendicular to the axis of the internal heat digestion tube. Each horizontal hole is equipped with a through pipe. A U-shaped heating wire is installed in each through pipe, and each internal heat digestion pipe corresponds to a switch and a temperature control seat. The temperature control seat is fixed in the middle of the internal heat digestion pipe, and each temperature control seat is equipped with a temperature sensor and two U U-shaped heating wire, temperature sensor and U-shaped heating wire are respectively connected to the electrical control box through wires, and several switches are installed on the upper surface of the cabinet, and the switches are connected to the control box through wires. The invention is useful for the determination of proteins in various biological and plant samples.
Owner:QIQIHAR UNIVERSITY
Hepatitis C virus ns5b RNA polymerase inhibitory polypeptide sequence and application thereof
ActiveCN104530189BFine and in-depth microcosmic researchReduce screening intensityPeptide/protein ingredientsPeptidesPharmaceutical SubstancesProtein measurement
The present invention designs a series of polypeptide combination libraries of different lengths, uses molecular docking software to carry out molecular docking of the polypeptides and specific inhibitory sites on HCV NS5B RNA polymerase, conducts comprehensive analysis on the results, and screens out HCV NS5B RNA inhibitors The polypeptide with polymerase activity lays the foundation for further research and development of anti-HCV drugs. The results of the binding experiment between the modified polypeptide and the expressed protein of NS5B show that: the designed polypeptide can bind to the expressed protein, and there is a correlation between the measurement result of the expressed fusion protein and the result of virtual docking. The polypeptide of the present invention can be used to detect the HCV NS5B protein. The results of polypeptide inhibition screening by the HCV NS5B RNA polymerase in vitro activity identification system show that the polypeptide of the present invention has a significant inhibitory effect on the polymerase activity of HCV NS5B protein, with an IC50 of up to 9.85 μM, and can be used as a potential drug for HCV inhibition.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Free protein S determination kit and preparation method thereof
InactiveCN114839387AHigh sensitivityEasy to operateDisease diagnosisBiological testingAntiendomysial antibodiesFree protein
The invention discloses a free protein S determination kit and a preparation method thereof. The kit comprises a reagent R1 and a reagent R2, the reagent R1 comprises a first buffer solution, a first stabilizer and a coagulant, and the pH value of the reagent R1 is 6.0-9.0; the R2 reagent comprises anti-human FPS antibody latex particles, a second buffer solution and a second stabilizer, the pH value of the R2 reagent is 6.0-9.0, and FPS is free protein S; the preparation method comprises the following steps: adding a first stabilizer and a coagulant into a first buffer solution, and adjusting the pH value to 6.0-9.0 to prepare a reagent R1; adding anti-human FPS antibody latex particles and a second stabilizer into a second buffer solution, and adjusting the pH value to 6.0-9.0 to prepare a reagent R2; according to the invention, the anti-human FPS antibody is coupled with the latex particles, and the FPS in the human plasma is determined by using the latex enhanced immunoturbidimetry, so that the accuracy is high, the stability is good, and the linear range is wide; the preparation method is simple and stable in process.
Owner:SHENZHEN DYMIND BIOTECH
Detection system for determining occupational fatigue degree of key industry based on human saliva protein
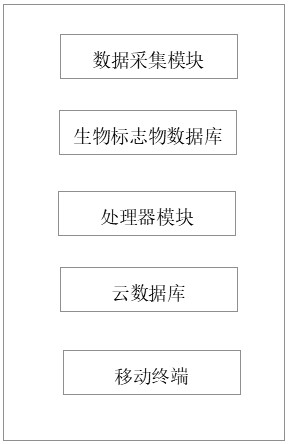
PendingCN113180595ARealize visual detectionMaterial analysis by electric/magnetic meansDisease diagnosisInformation processingHuman body
The invention discloses a detection system for determining the occupational fatigue degree of key industries based on human saliva protein, and relates to the technical field of fatigue detection. The fatigue detection system comprises a biomarker database, a data acquisition module, a processor module, a cloud database and a mobile terminal, network visual detection is realized by establishing a fatigue crowd biomarker database, a front-end saliva fatigue biomarker parameter acquisition and information processing system is applied to carry out monitoring, the physical quantity data such as the type content of fatigue biomarkers are collected and monitored, the processor module carries out processing and function analysis on the collected data signals, a man-machine interaction interface is established, a fatigue prevention and control technology network system is constructed through cloud data, the data are sent to the monitoring terminal through a network, the fatigue degree of the occupational population is prejudged by prevention and control personnel, prevention and control measures are taken, and a prevention and control system covering links of health management, fatigue detection, risk assessment, alarm intervention and the like is constructed.
Owner:HEBEI UNIV OF ENG
Hepatitis C virus NS5B RNA polymerase inhibitory polypeptide sequence and application thereof
ActiveCN104530189AFine and in-depth microcosmic researchReduce screening intensityPeptide/protein ingredientsPeptidesProtein measurementHepatitis viral c
The invention designs a series of polypeptide combinational libraries with different lengths. Polypeptides with functions of inhibiting the activity of HCV NS5B RNA polymerase can be screened out by performing molecular docking on the polypeptides and specific inhibitory sites on HCV NS5B RNA polymerase by using molecular docking software and performing comprehensive analysis on results, and can be used for laying a foundation for further researching and developing anti-HCV medicines. Combined experimental results of modified polypeptide and NS5B expression protein show that the designed polypeptides can be combined with expression protein to express that fusion protein measurement results have correlations with virtual docking results. The polypeptides designed by the invention can be used for detecting HCV NS5B protein, and results obtained by performing polypeptide inhibition screening by using an HCV NS5B RNA polymerase in-vitro activity identification system show that the polypeptides designed by the invention have significant inhibition effects on the polymerase activity of HCV NS5B protein, IC50 of the polypeptides can reach 9.85 microns, and the polypeptides can be used as potential medicines for inhibiting HCV.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Method for Determination of Absorption and Transport Amounts of Six Components of Baijizhong in Caco-2 Cell Model
ActiveCN105954411BEvaluation of in vivo absorption propertiesComponent separationAdenocarcinoma colonMedicine
The invention discloses a determination method for the absorption and transportation amount of six components in rhizoma bletillae in a Caco-2 cell model. The method comprises the steps of: preparing a rhizoma bletillae extract solution, a standard solution serving as a reference substance and an internal standard solution; establishing a human-derived colon adenocarcinoma cell line Caco-2 cell model; preparing a cell suspension through a Caco-2 cell model; determining the content of the six components by UPLC-MS / MS; determining the total protein content according to a Coomassie brilliant blue dye liquor protein determination kit method, and calculating the cell uptake X=the total protein of a to-be-determined substance. The invention adopts UPLC-MS / MS to establish the analysis method for the 6 components in a rhizoma bletillae extract, determines the influence of the rhizoma bletillae extract to absorption and uptake of Caco-2 cells under the conditions of time, concentration, temperature, pH and P-gh inhibitors, preliminarily evaluates the in vivo absorption characteristics of the rhizoma bletillae extract, and provides scientific basis for the research and development of the oral preparation of the rhizoma bletillae extract.
Owner:GUIZHOU MEDICAL UNIV
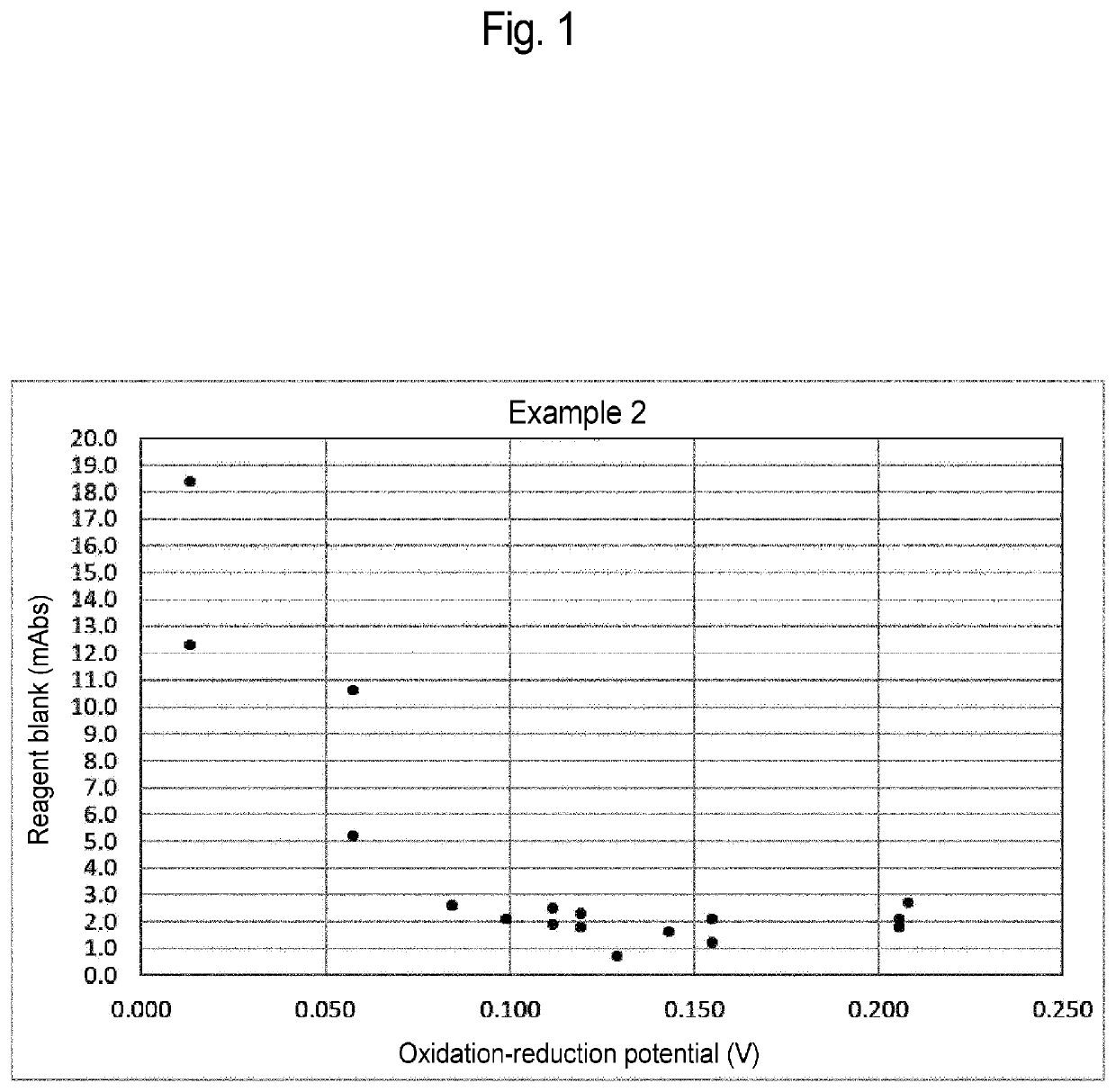
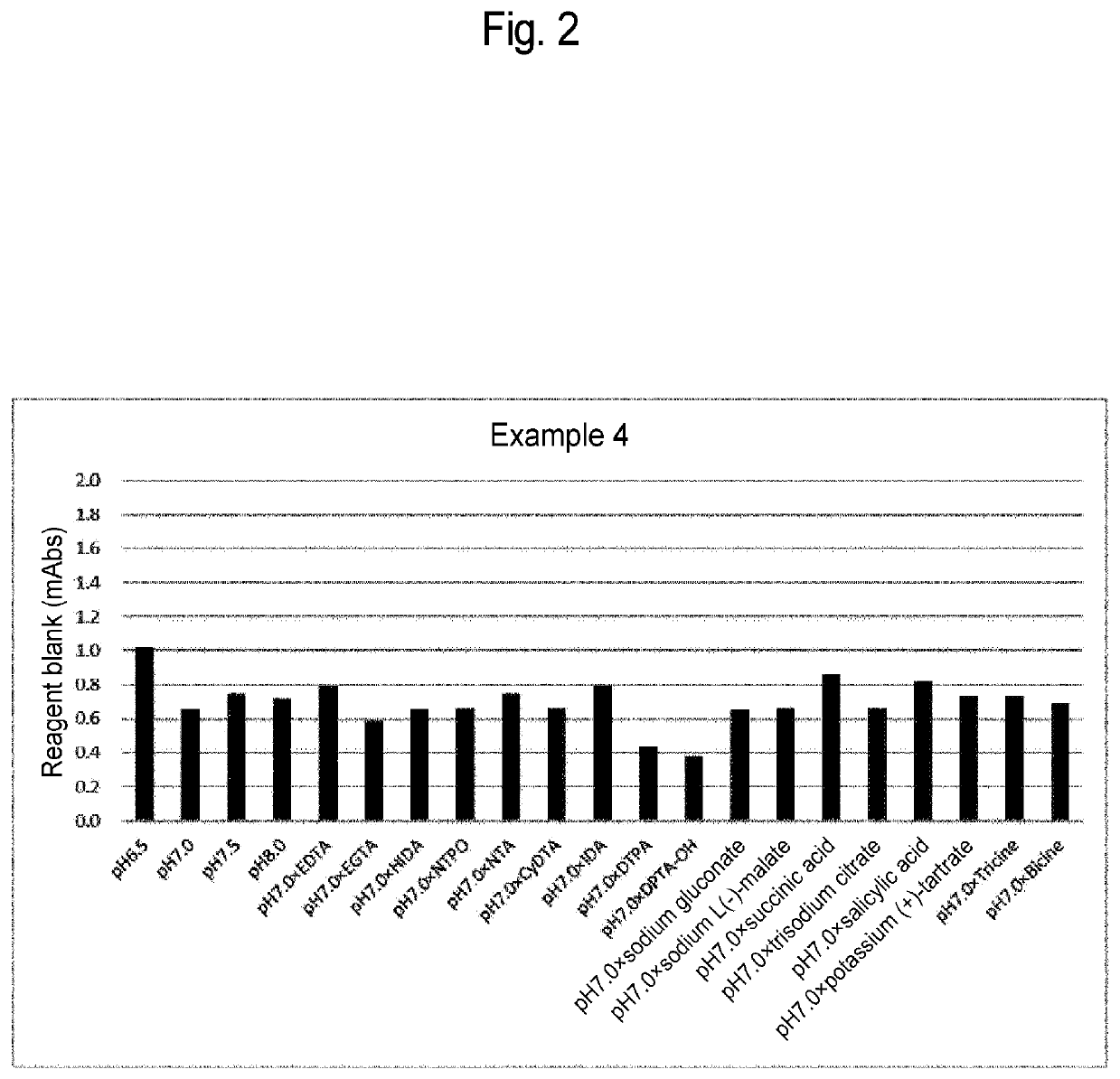
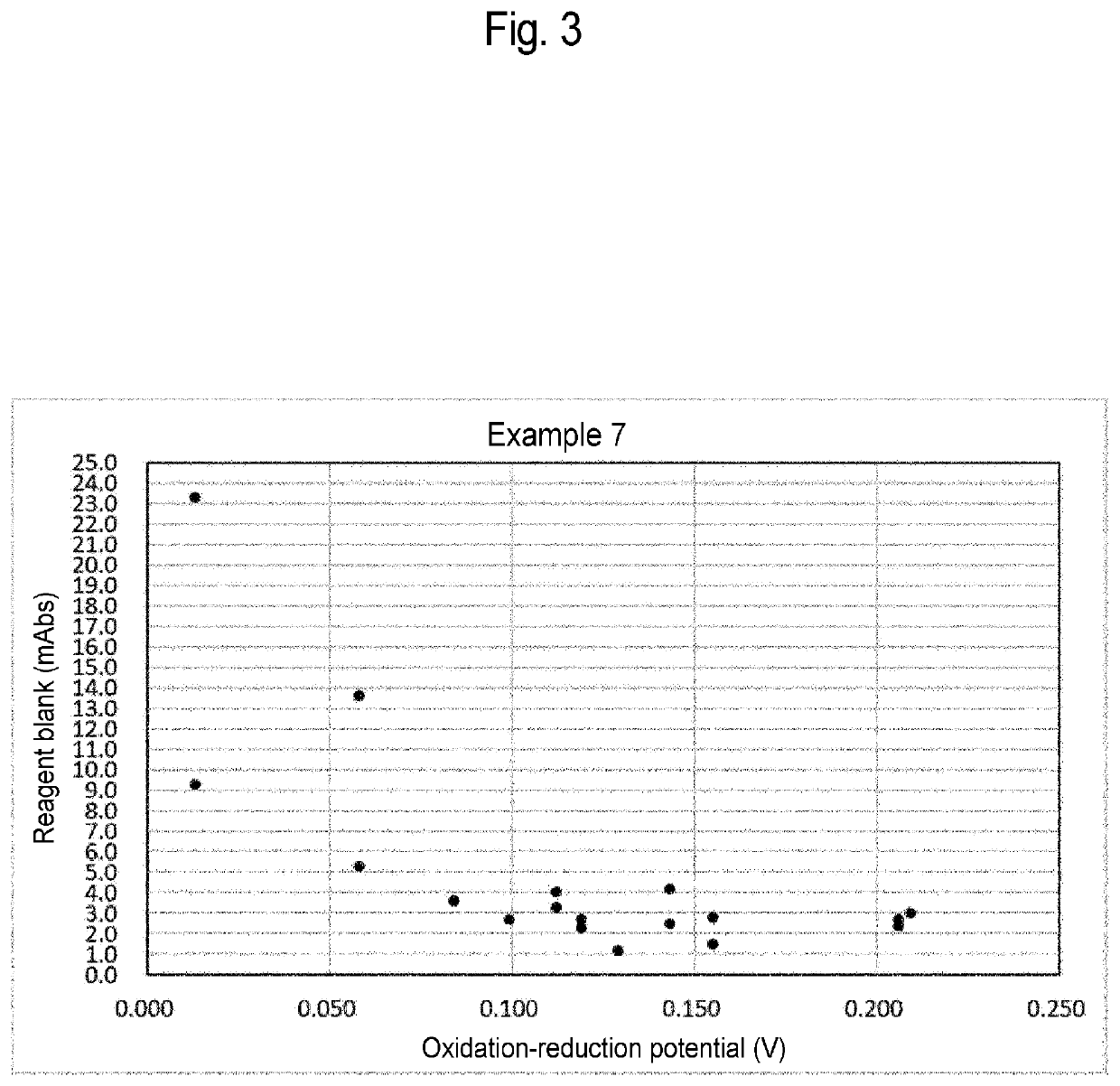
Glycated protein assay reagent containing stabilizer of protease that increases oxidation-reduction potential of ferrocyanide, method for assaying glycated protein, method for preserving glycated protein assay reagent, and method for stabilizing glycated protein assay reagent
PendingUS20220357334A1Suppresses elevationMicrobiological testing/measurementBiological testingCyanide compoundBiochemistry
Provided is a glycated protein assay reagent containing at least a Trinder reagent, 4-aminoantipyrine, protease, a stabilizer of the protease, and ferrocyanide, wherein at least the Trinder reagent is contained in a Trinder reagent-containing partial composition, at least the 4-aminoantipyrine is contained in a 4-aminoantipyrine-containing partial composition, the stabilizer of the protease is a stabilizer that increases the oxidation-reduction potential of the ferrocyanide above 0.058 V when the stabilizer of the protease and the ferrocyanide are mixed, and the oxidation-reduction potential is an oxidation-reduction potential in a reaction system containing the stabilizer of the protease and the ferrocyanide and not containing glycated protein.
Owner:ASAHI KASEI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com