Patents
Literature
33 results about "Colon adenocarcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
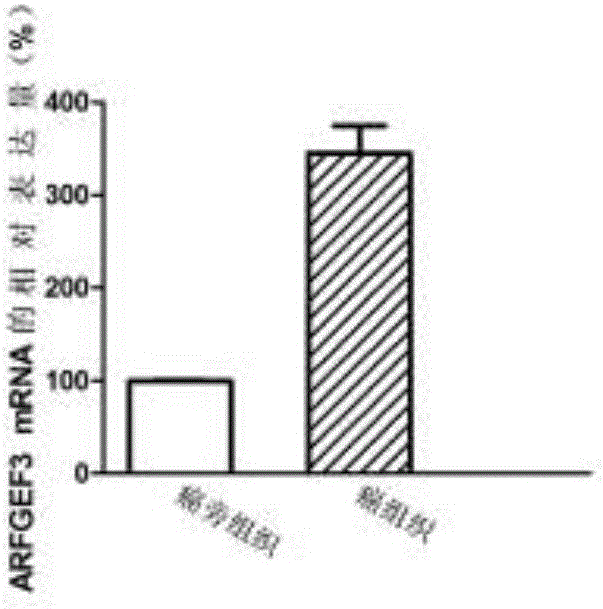
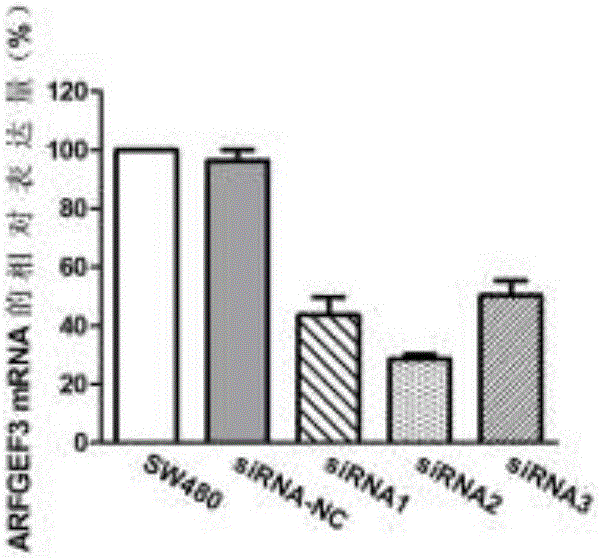
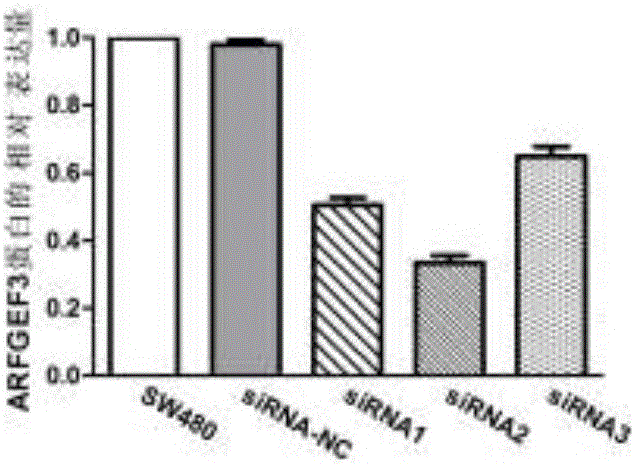
Application of gene used as biomarker in colon adenocarcinoma
InactiveCN107177666AAchieve early diagnosisReduced expression levelMicrobiological testing/measurementBiological testingOncologyBiomarker (petroleum)
The invention discloses application of a gene used as a biomarker in colon adenocarcinoma. It first discovered that proliferation, migration and invasion of colon adenocarcinoma cells can be changed by increasing expression of an ARFGEF3 gene in a patient suffering from the colon adenocarcinoma and reducing the expression level of ARFGEF3, and therefore, ARFGEF3 can be used for developing a product for early diagnosis of the colon adenocarcinoma and a drug for treating the colon adenocarcinoma. The application of the gene used as the biomarker in the colon adenocarcinoma provides a theory and experiment foundation for implementation of the precision medicine.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Building method of colorectal cancer neostomy orthotopic transplantation model
InactiveCN101015700ASimplify the build processMeet the requirements of ethicsIn-vivo testing preparationsLymphatic SpreadWilms' tumor
The invention provides a method for establishing orthotopic transplantation model of colorectal cancer, which comprises a) selecting and culturing colon adenocarcinoma cell line and preparing cell suspension; b) performing colostomy on living experimental animal; c) and inoculating the above colon adenocarcinoma cell suspension to submucosal stroma to allow natural growth. The biological traits such as route of metastasis of the transplanted adenocarcinoma cells are similar to that of the primary colon adenocarcinoma cell. The invention is advantageous in that the model is easy to be established, the transplanted colon adenocarcinoma locates at body surface so that it can be sampled and observed conveniently to meet the requirement for dynamic research, and the consumption of experimental animals can be reduced.
Owner:NANJING HOSPITAL OF T C M
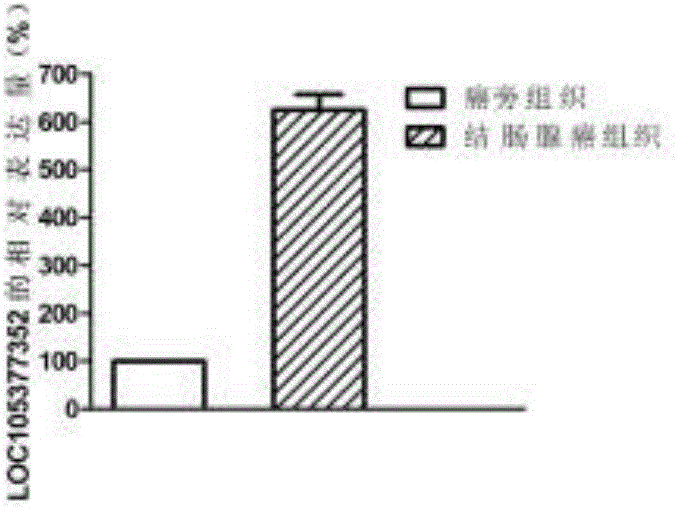
Molecular marker for colon adenocarcinoma
ActiveCN106676191AImprove the quality of lifeFacilitate early detectionMicrobiological testing/measurementMaterial analysisMedicineTrue positive rate
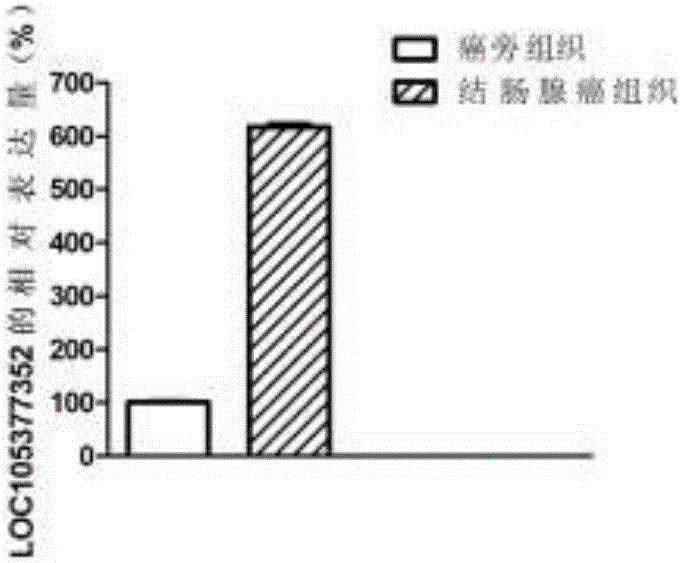
The invention discloses a molecular marker for colon adenocarcinoma. The molecular marker is LOC105377352, wherein the expression of the LOC105377352 in a patient of the colon adenocarcinoma is increased. The lncRNA (long non-coding Ribonucleic Acid) marker provided by the invention can be used for early diagnosis of the colon adenocarcinoma and has relatively high sensitivity and specificity.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
Determination method for absorption and transportation amount of six components in rhizoma bletillae in Caco-2 cell model
ActiveCN105954411AEvaluation of in vivo absorption propertiesComponent separationInternal standardIn vivo absorption
The invention discloses a determination method for the absorption and transportation amount of six components in rhizoma bletillae in a Caco-2 cell model. The method comprises the steps of: preparing a rhizoma bletillae extract solution, a standard solution serving as a reference substance and an internal standard solution; establishing a human-derived colon adenocarcinoma cell line Caco-2 cell model; preparing a cell suspension through a Caco-2 cell model; determining the content of the six components by UPLC-MS / MS; determining the total protein content according to a Coomassie brilliant blue dye liquor protein determination kit method, and calculating the cell uptake X=the total protein of a to-be-determined substance. The invention adopts UPLC-MS / MS to establish the analysis method for the 6 components in a rhizoma bletillae extract, determines the influence of the rhizoma bletillae extract to absorption and uptake of Caco-2 cells under the conditions of time, concentration, temperature, pH and P-gh inhibitors, preliminarily evaluates the in vivo absorption characteristics of the rhizoma bletillae extract, and provides scientific basis for the research and development of the oral preparation of the rhizoma bletillae extract.
Owner:GUIZHOU MEDICAL UNIV
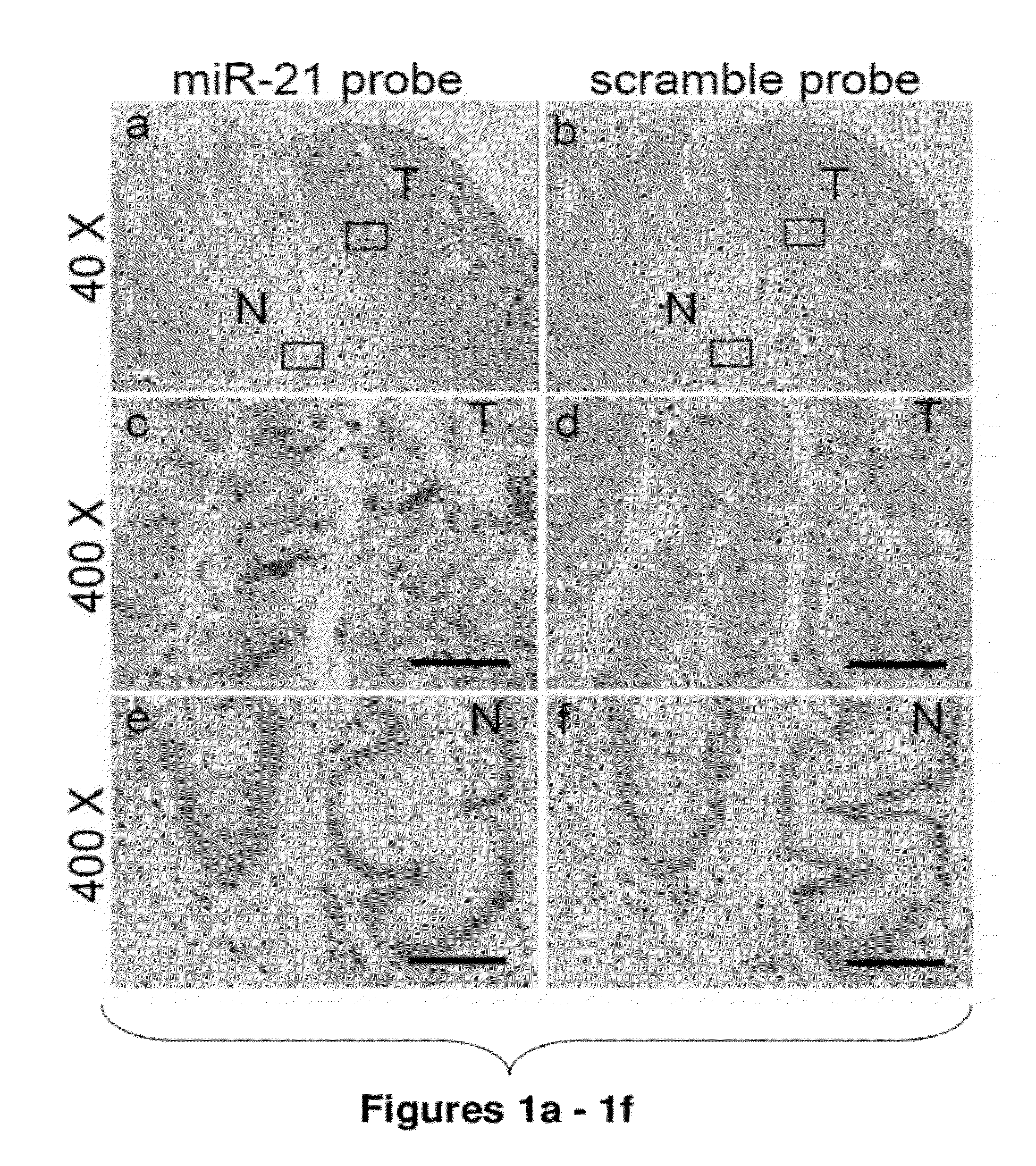
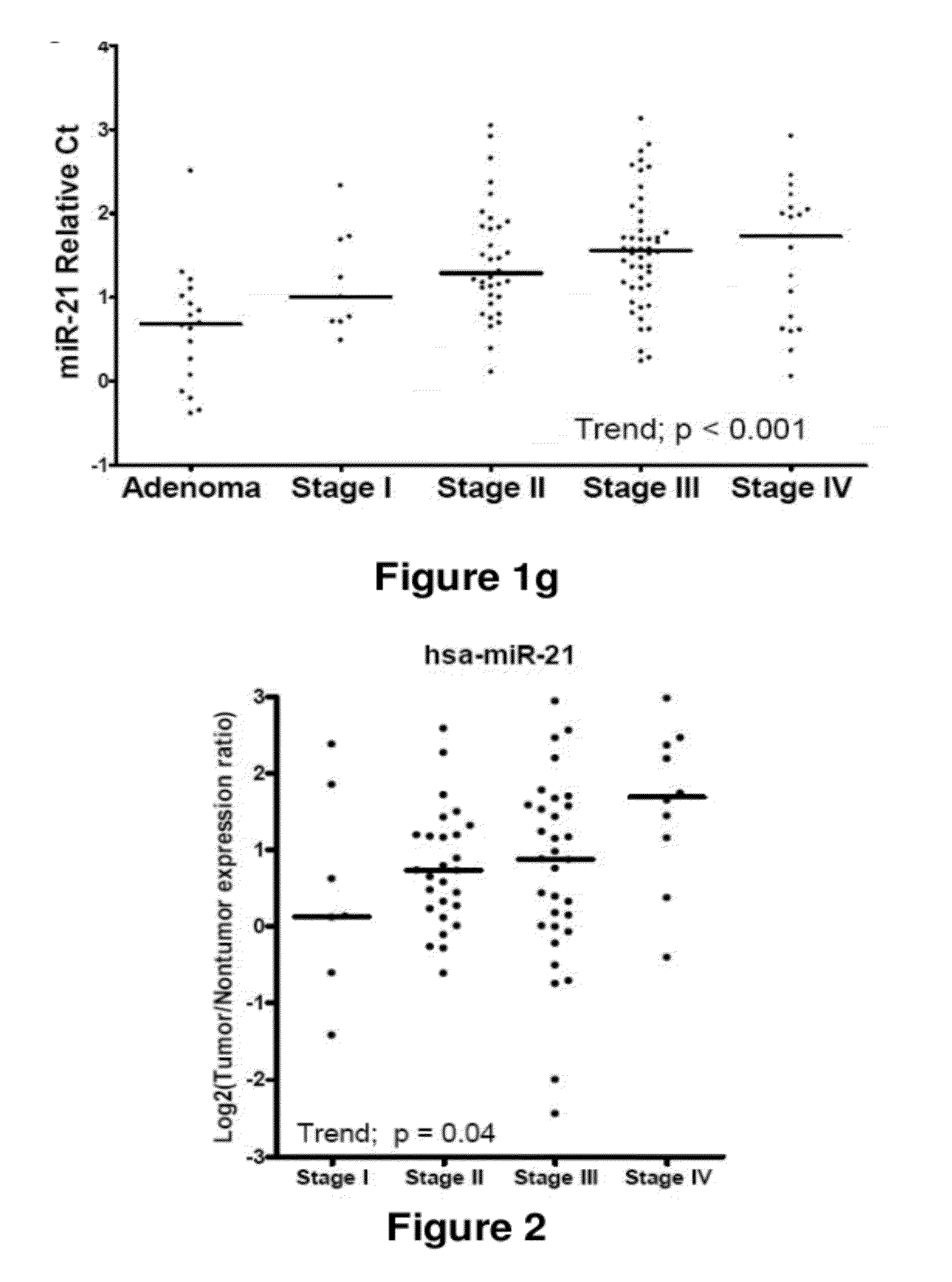
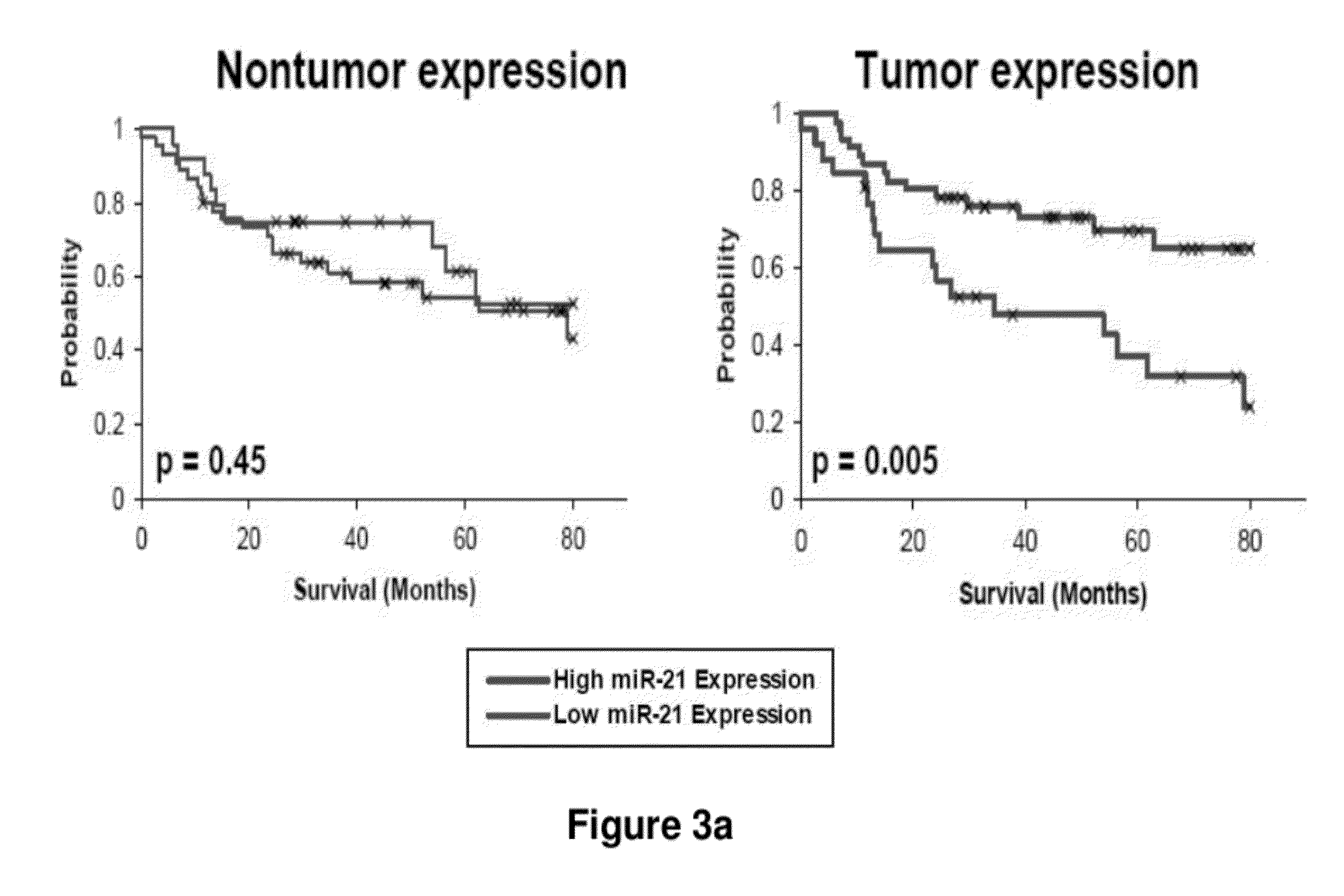
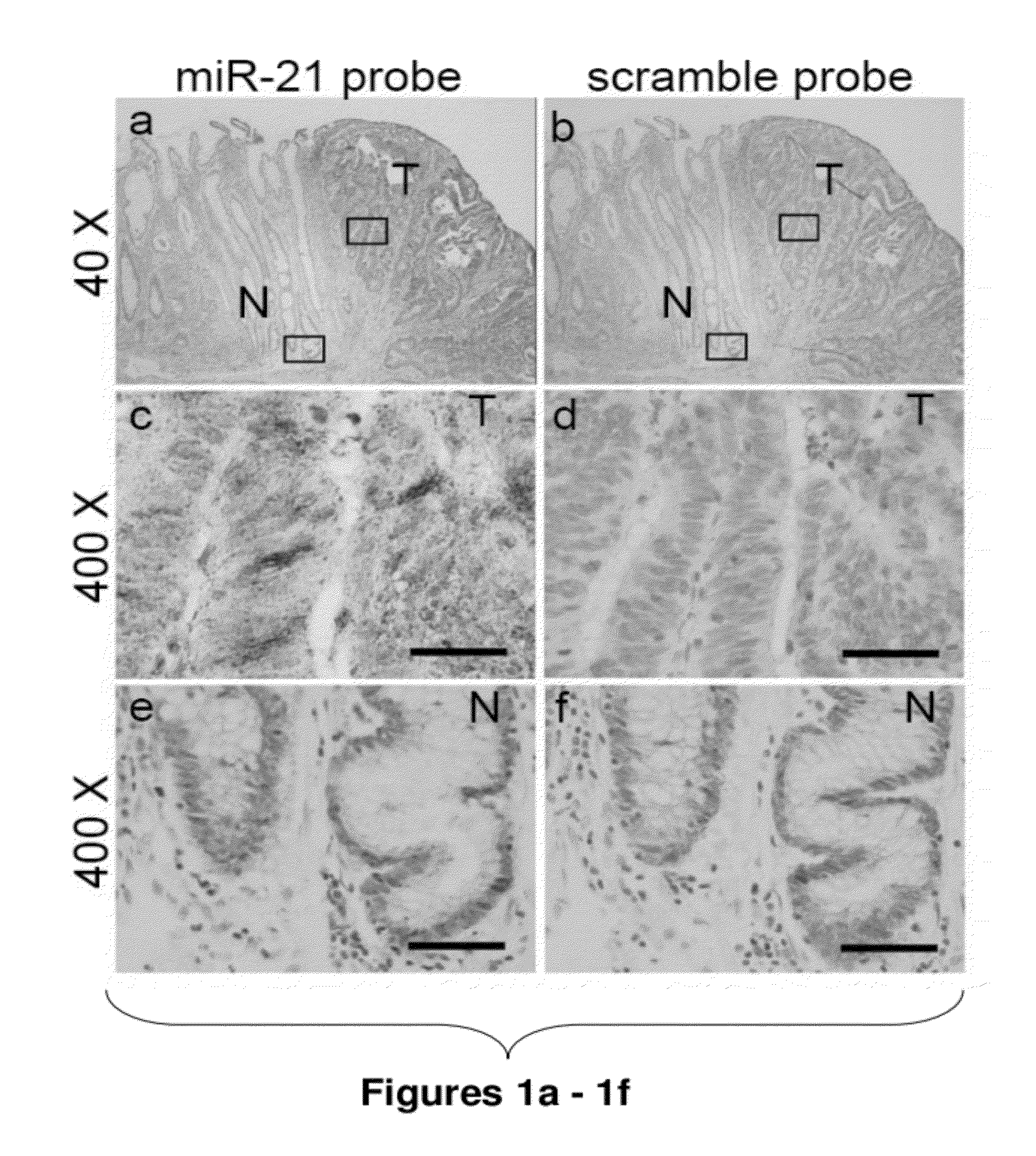
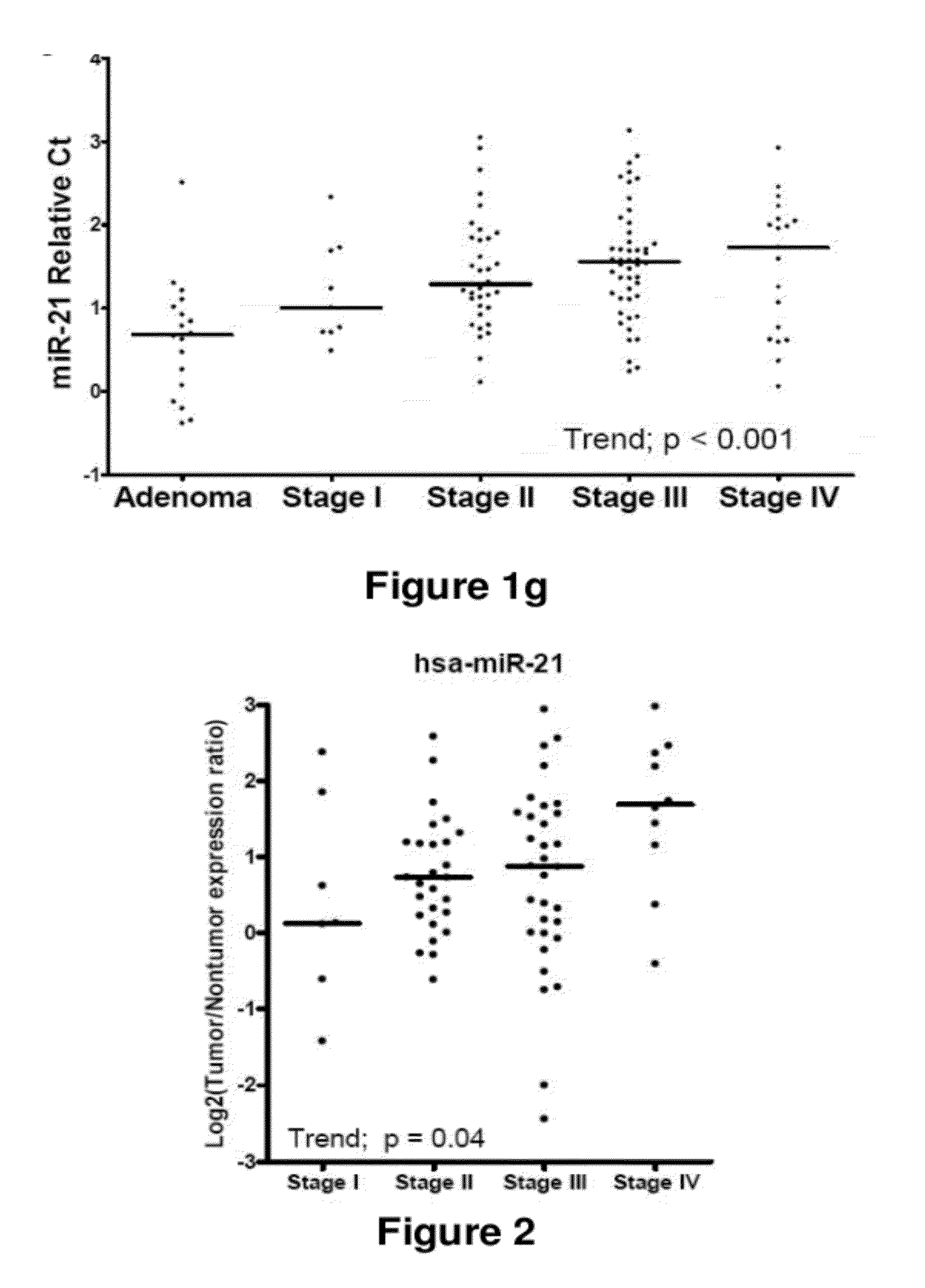
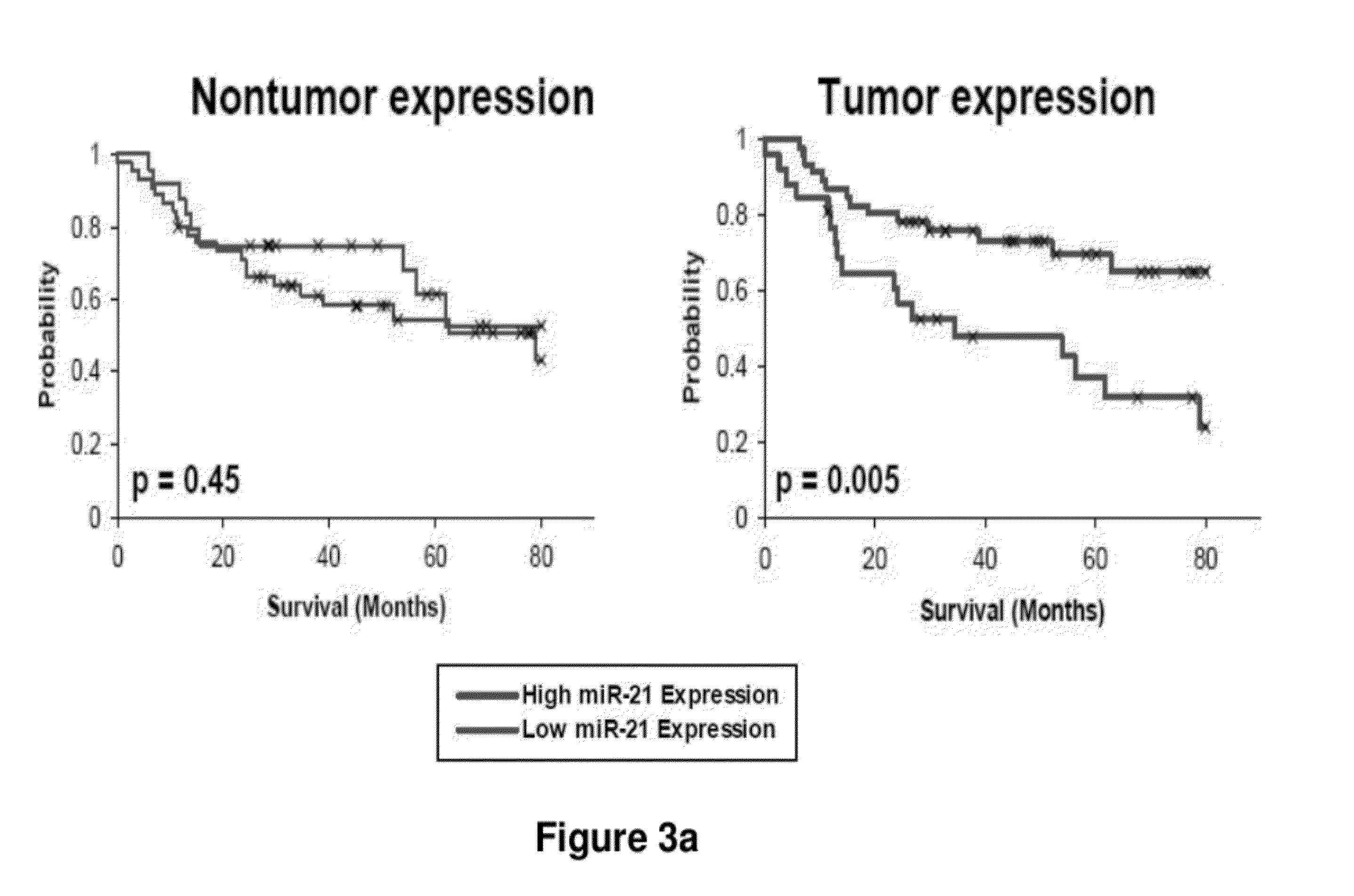
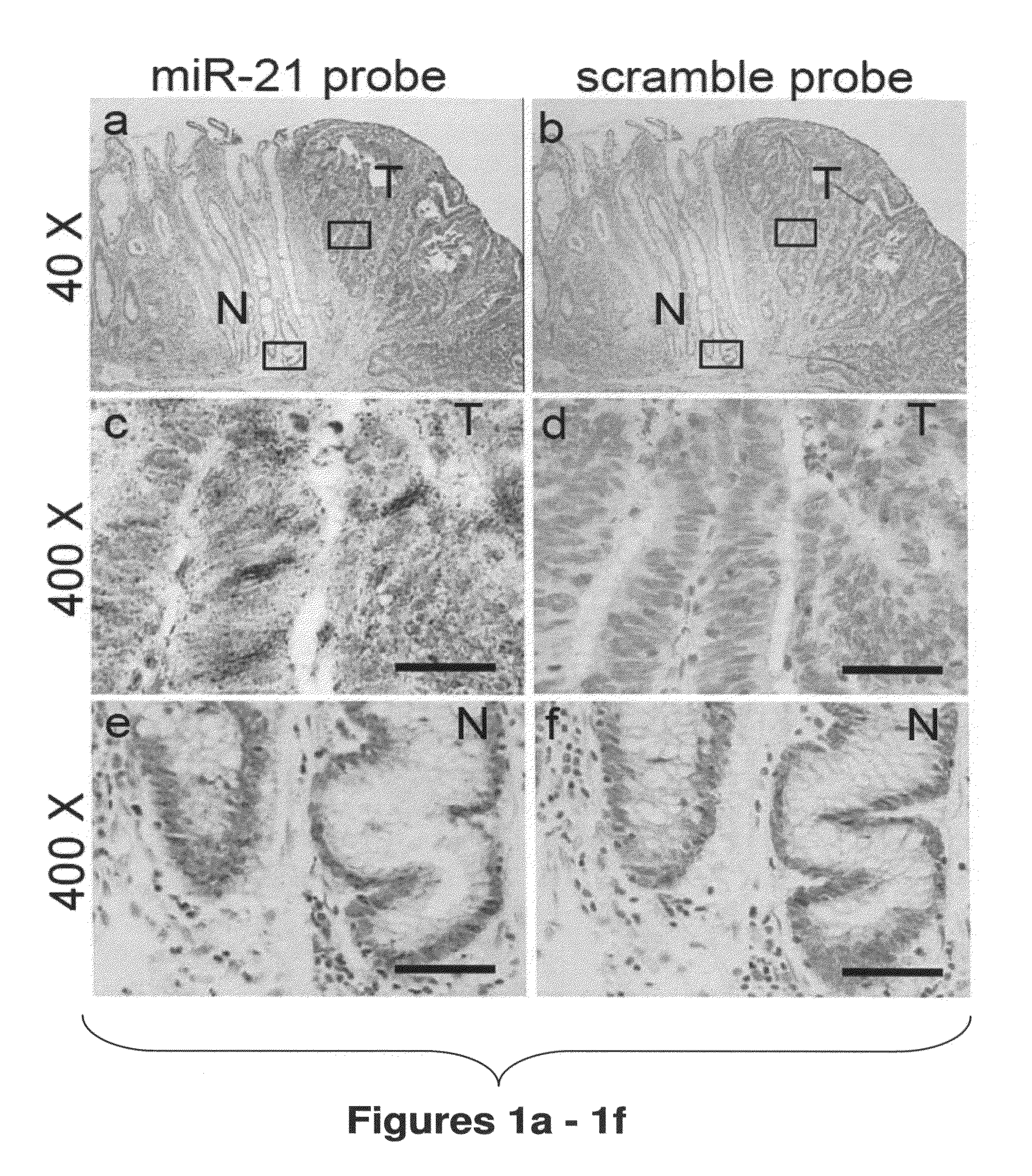
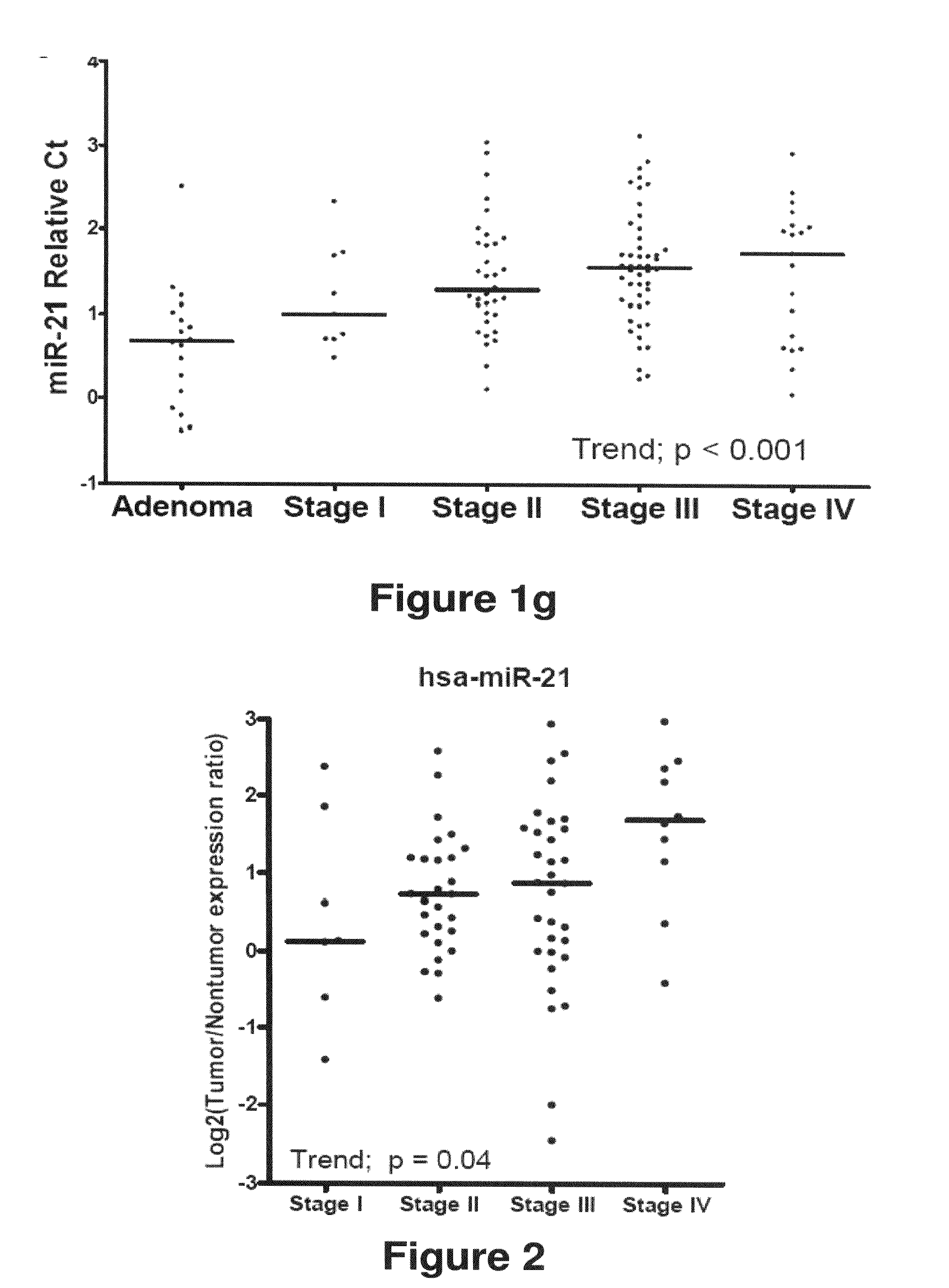
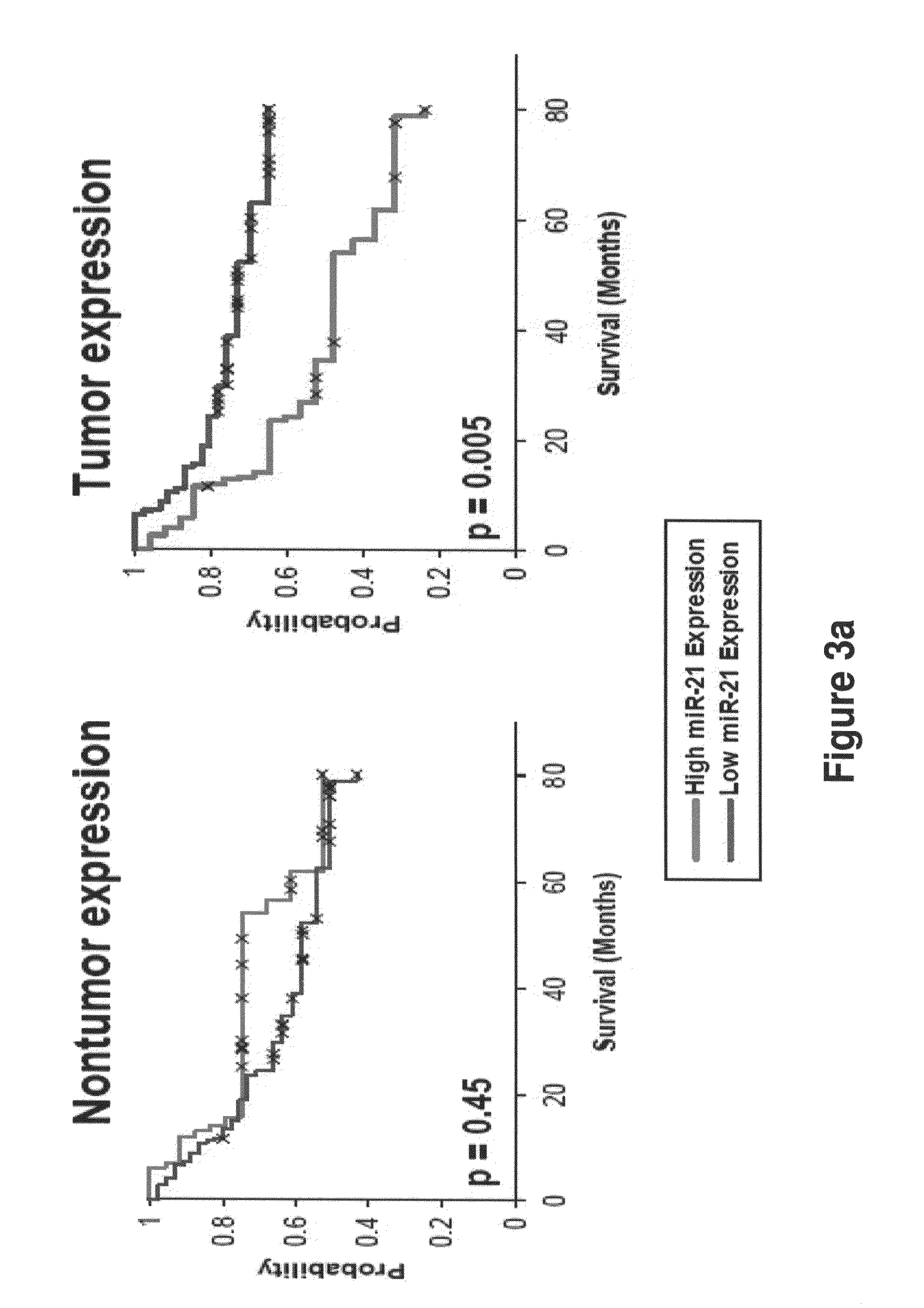
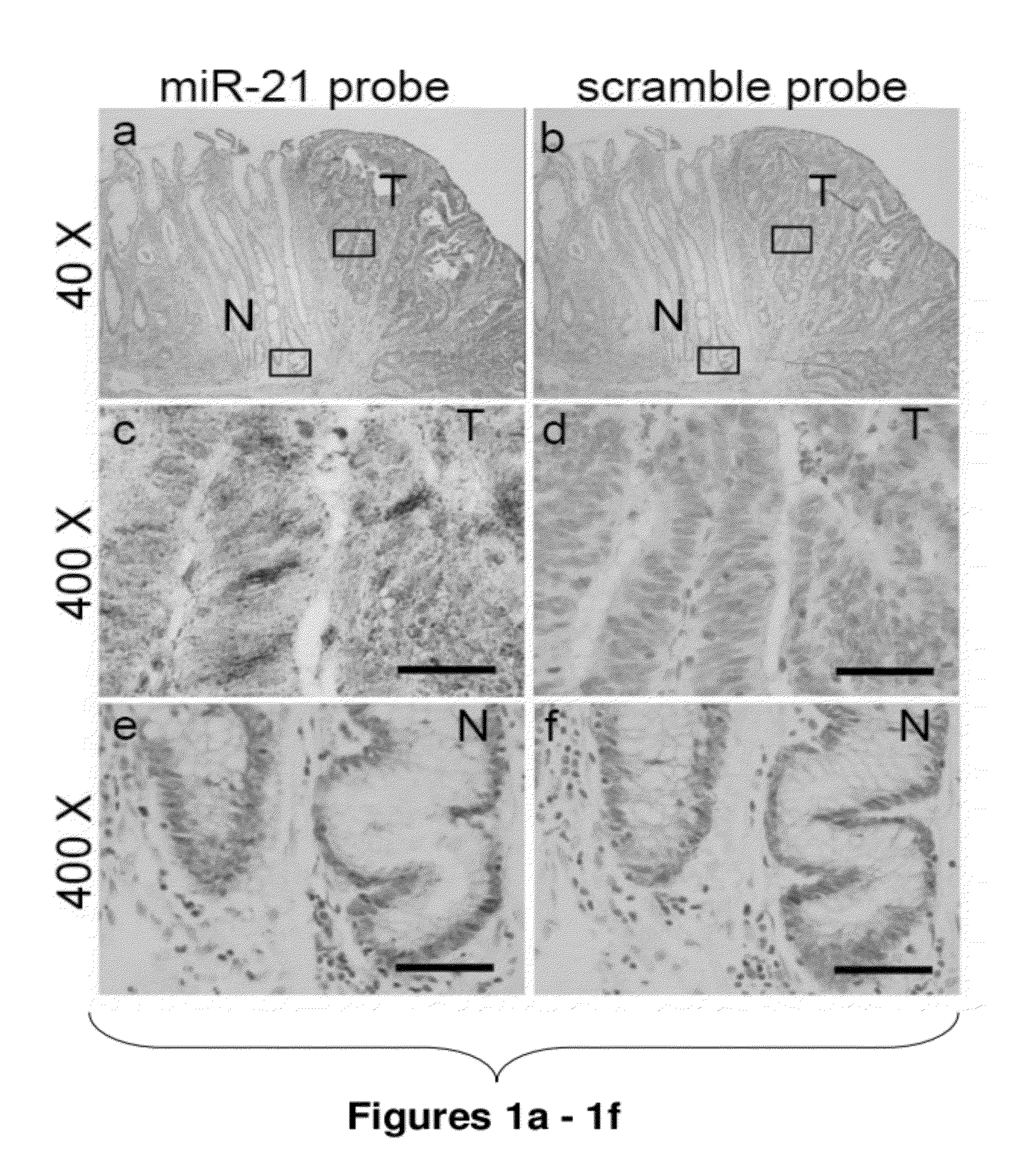
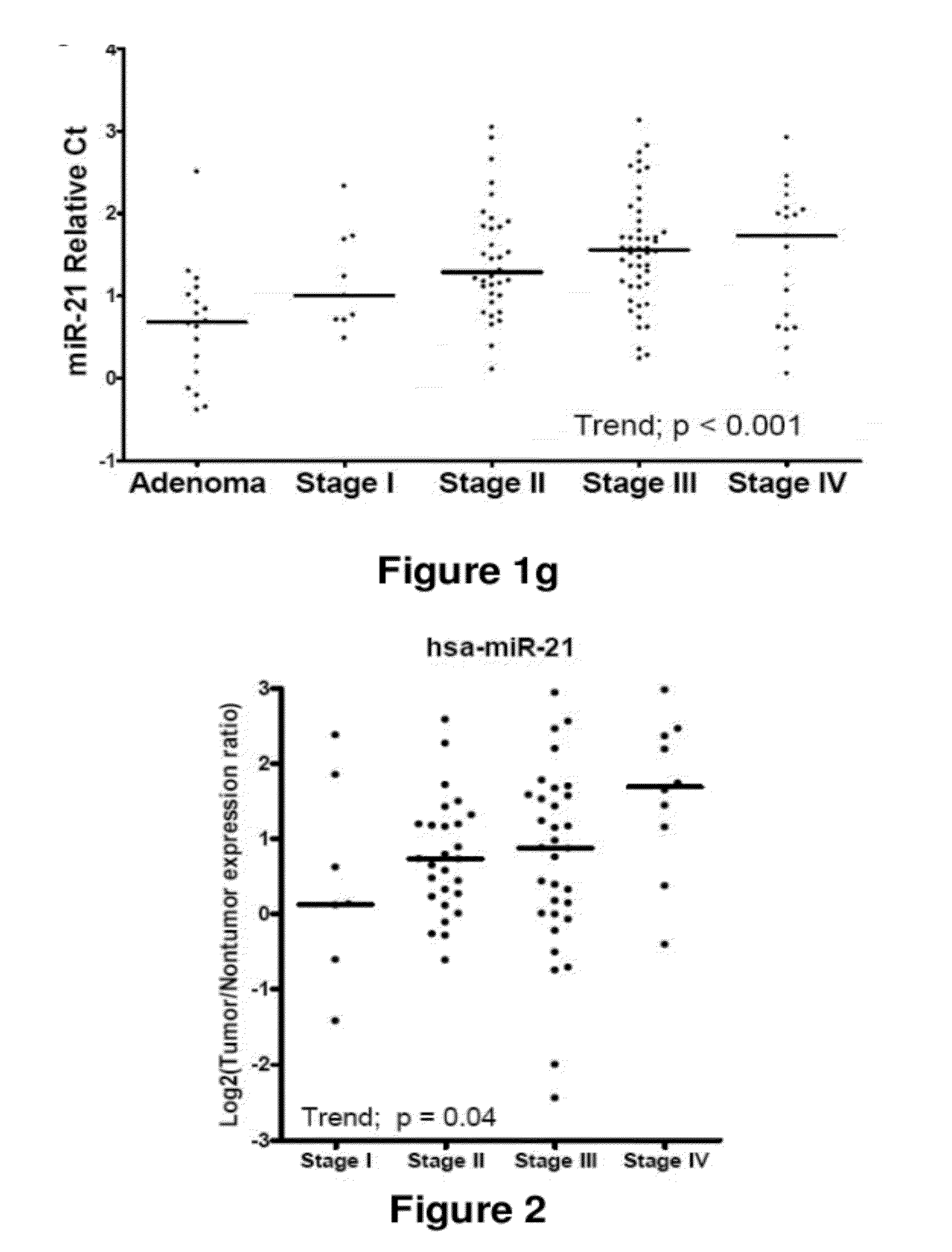
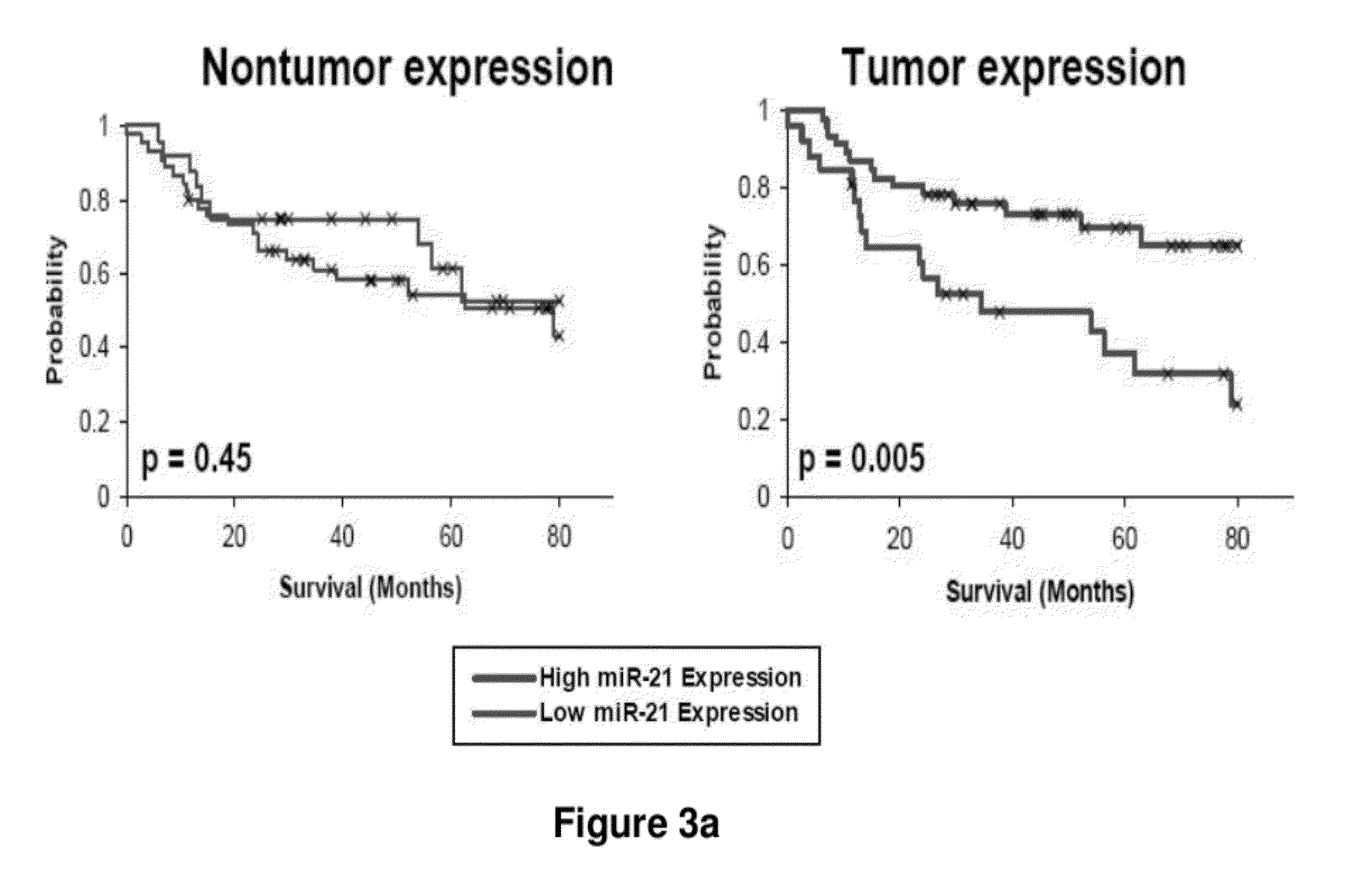
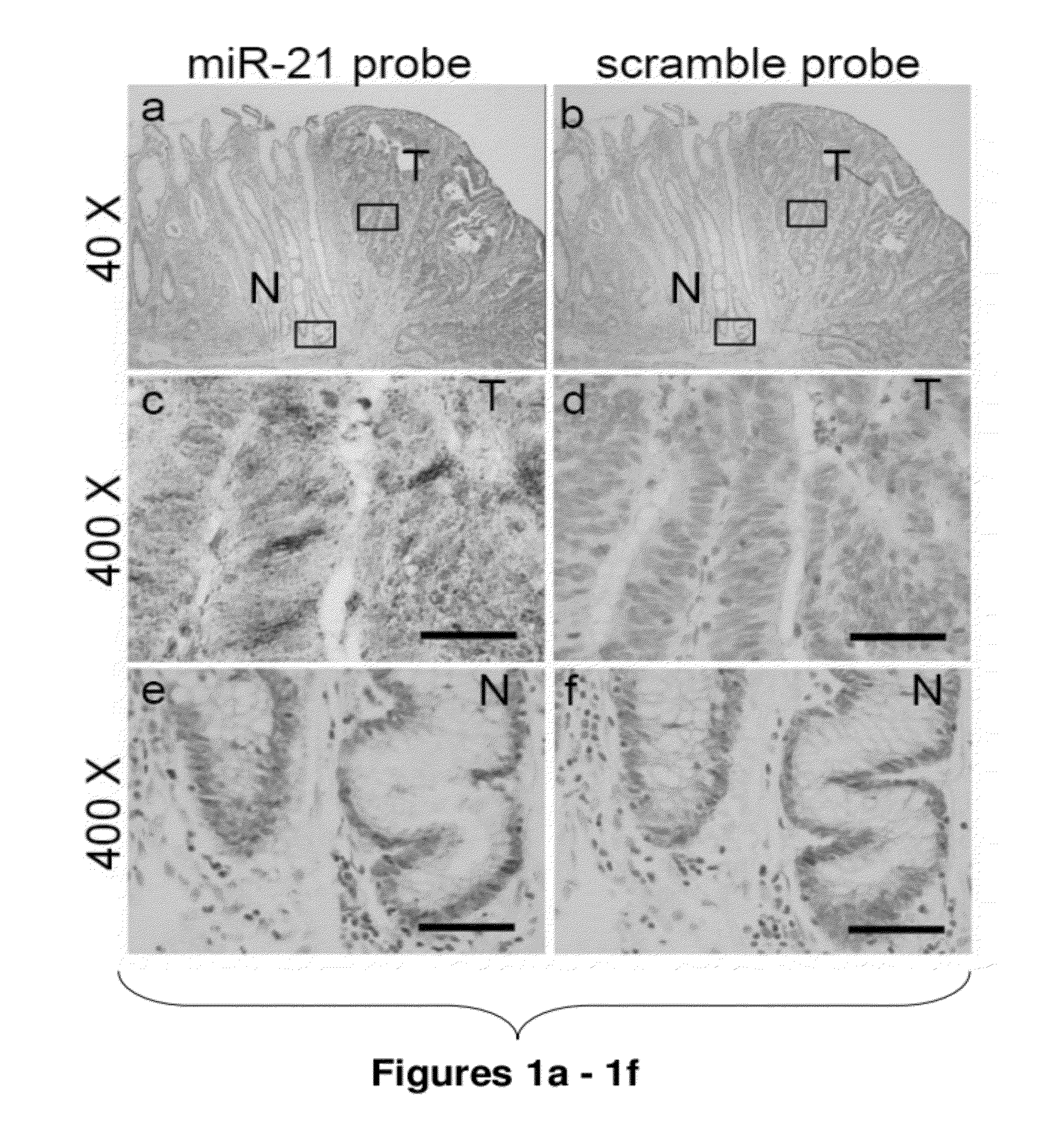
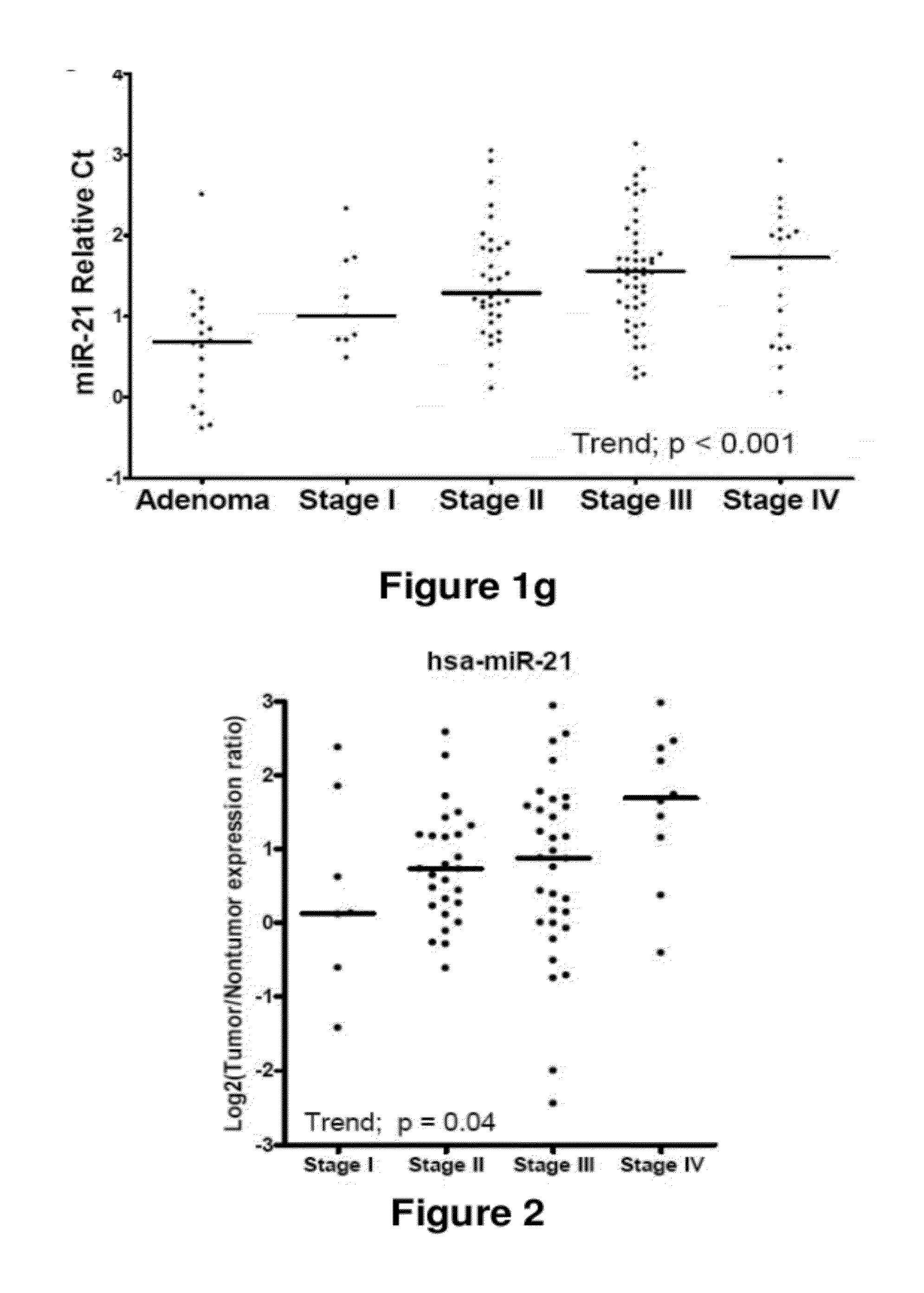
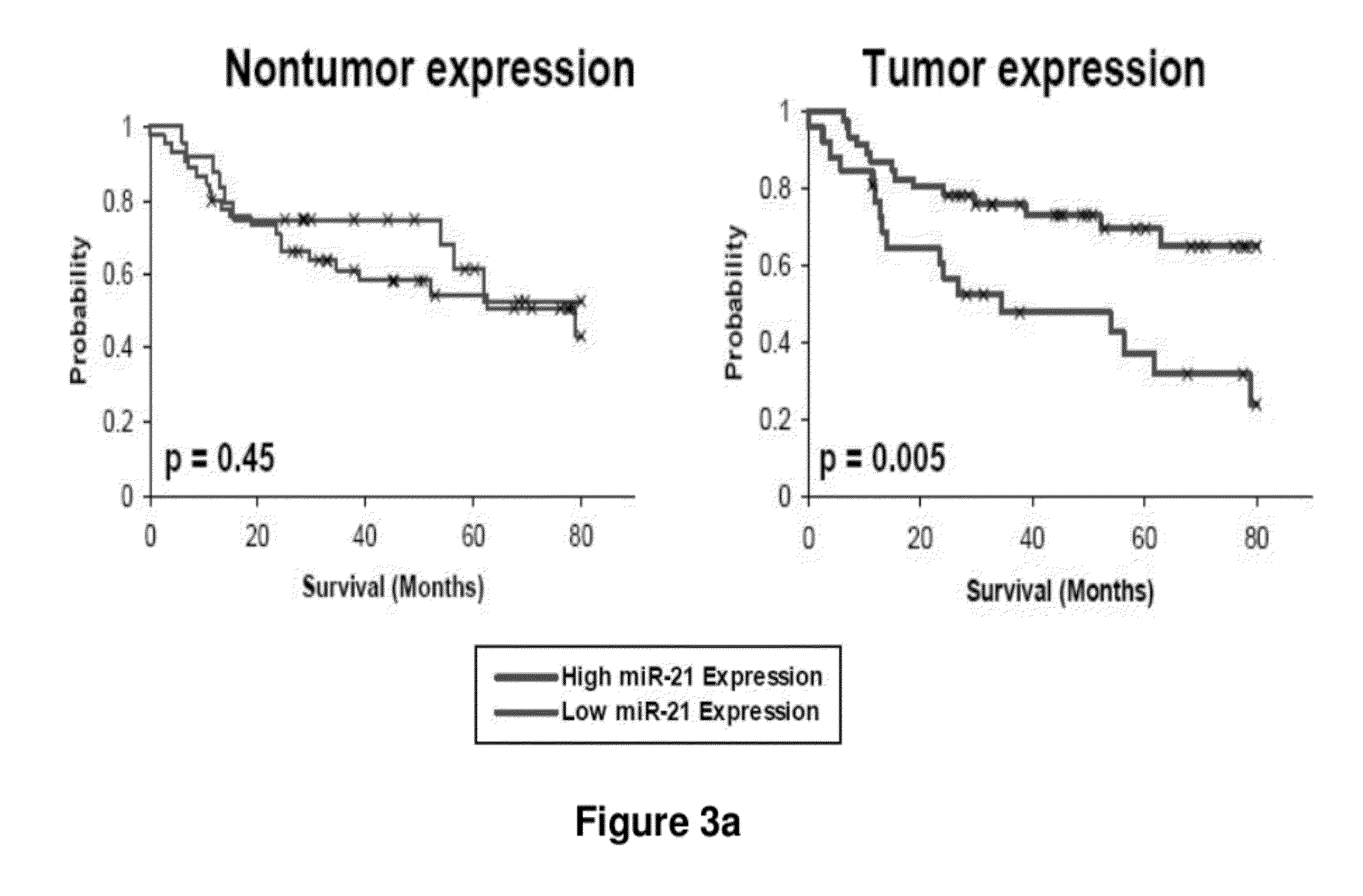
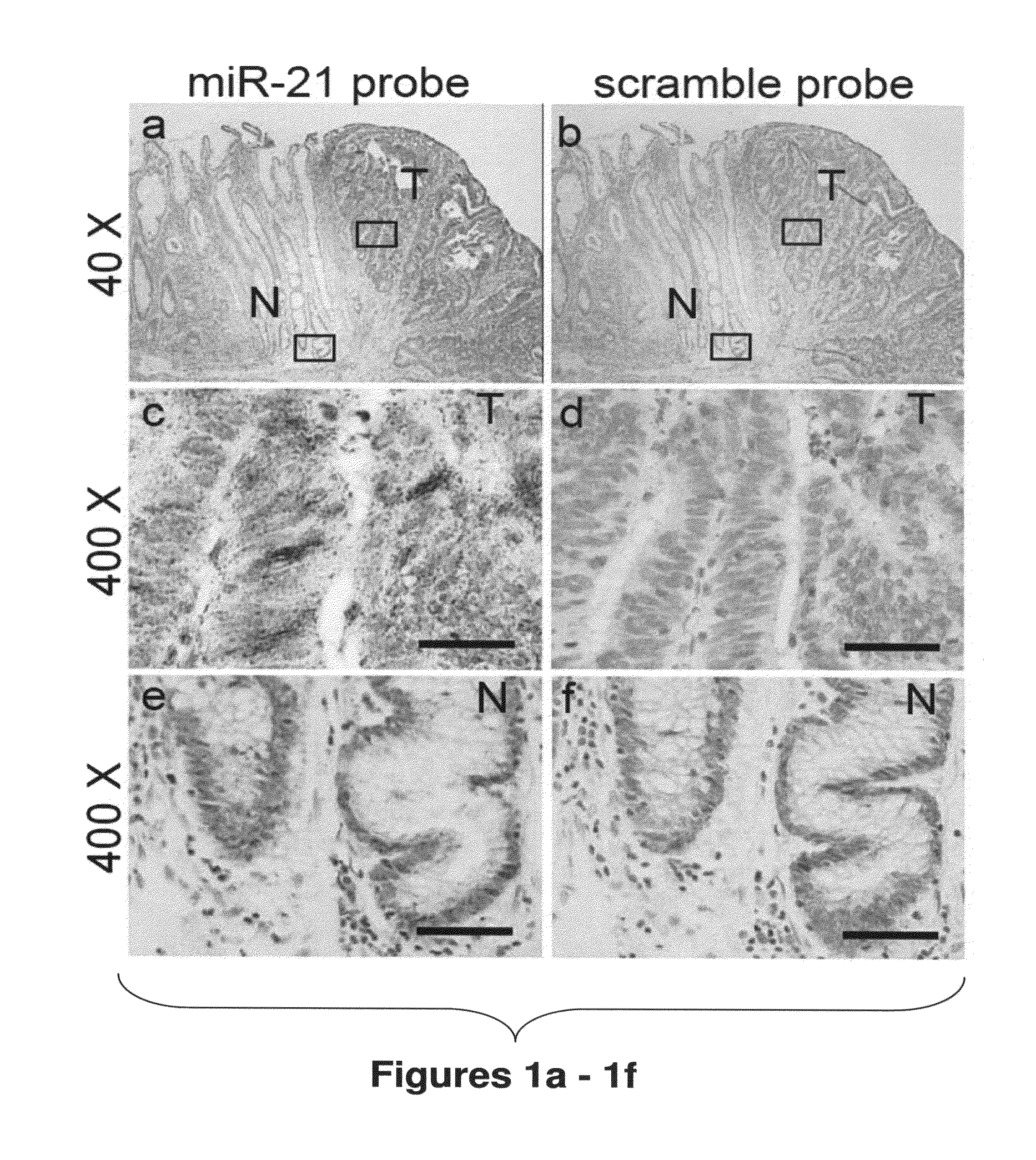
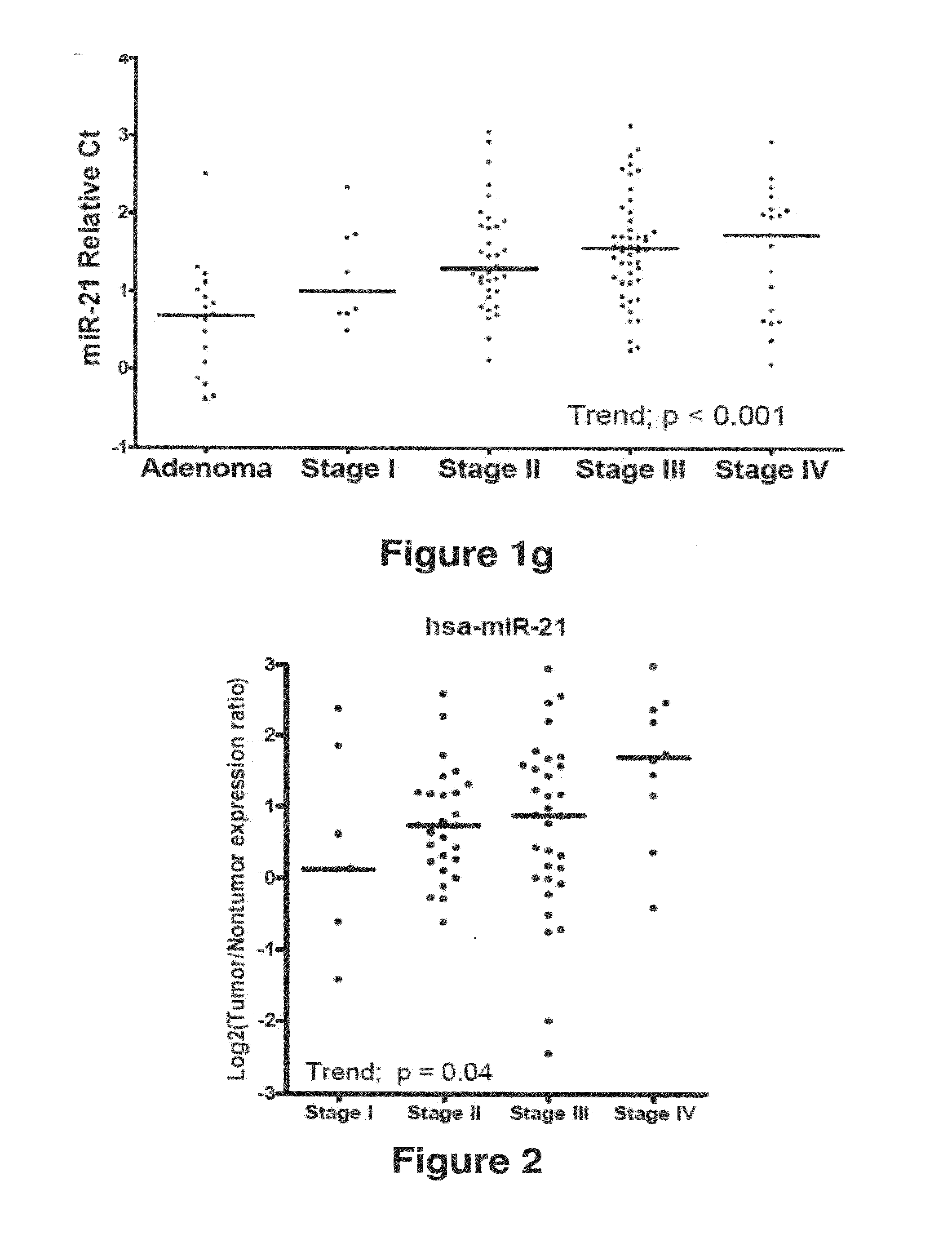
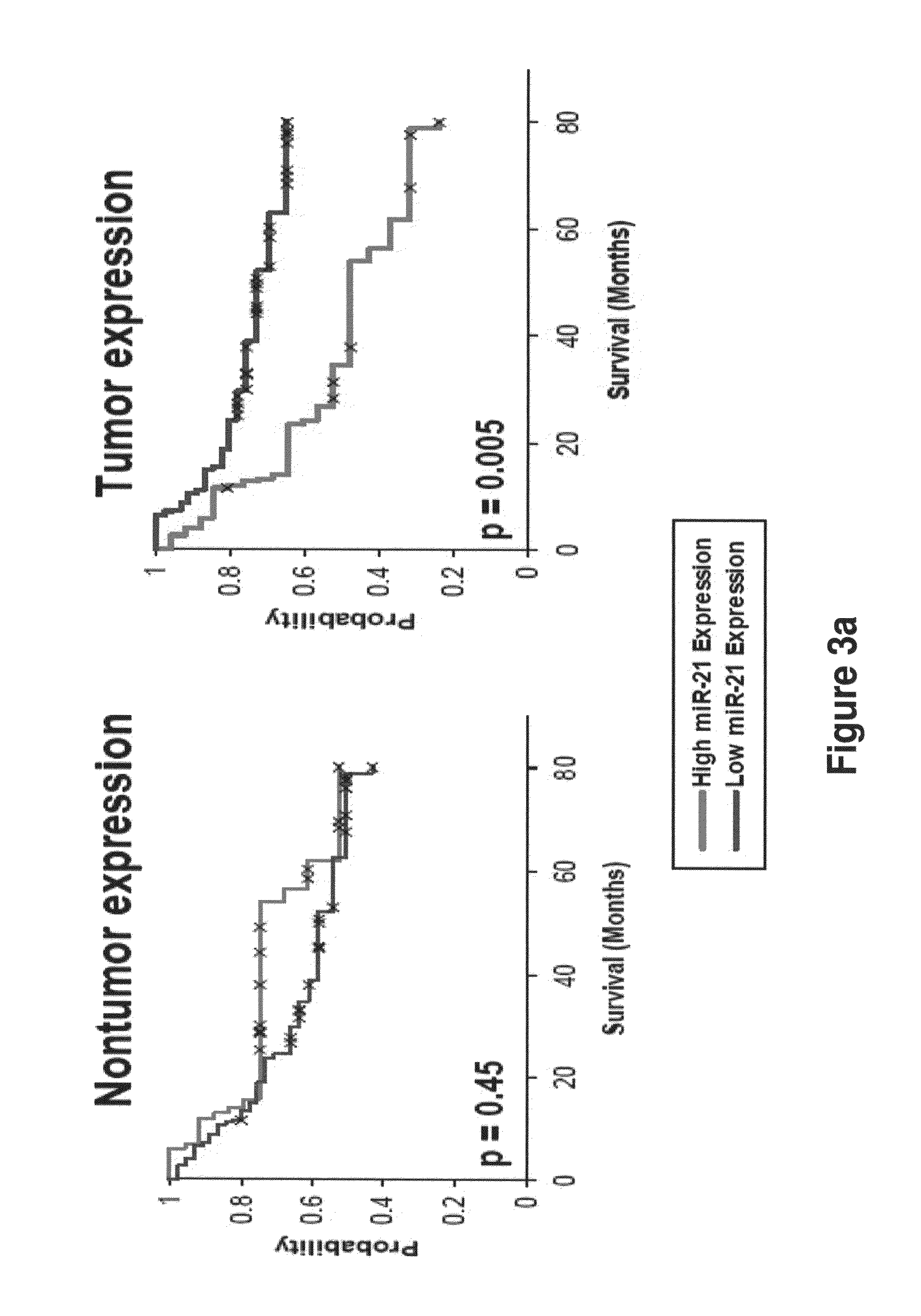
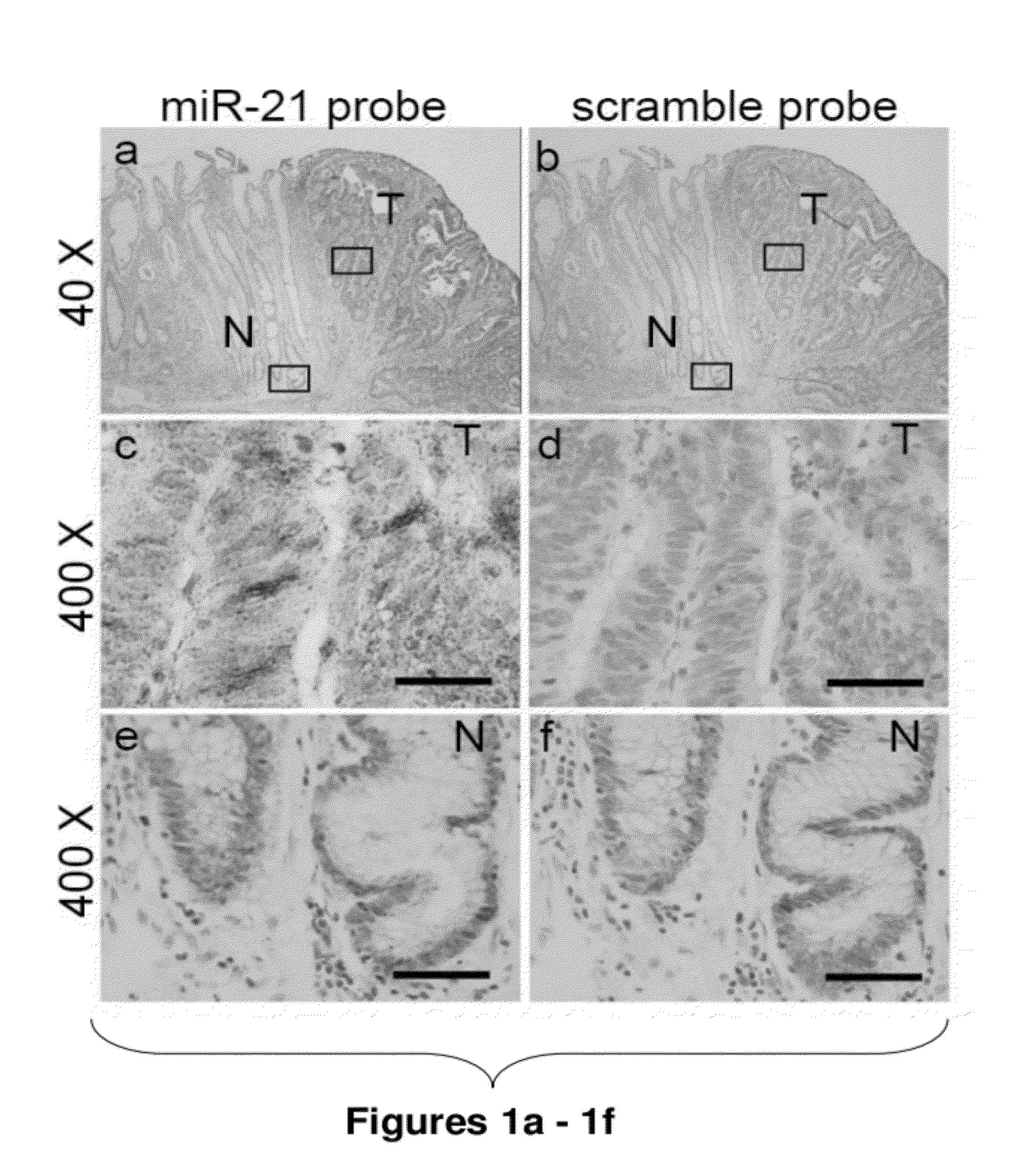
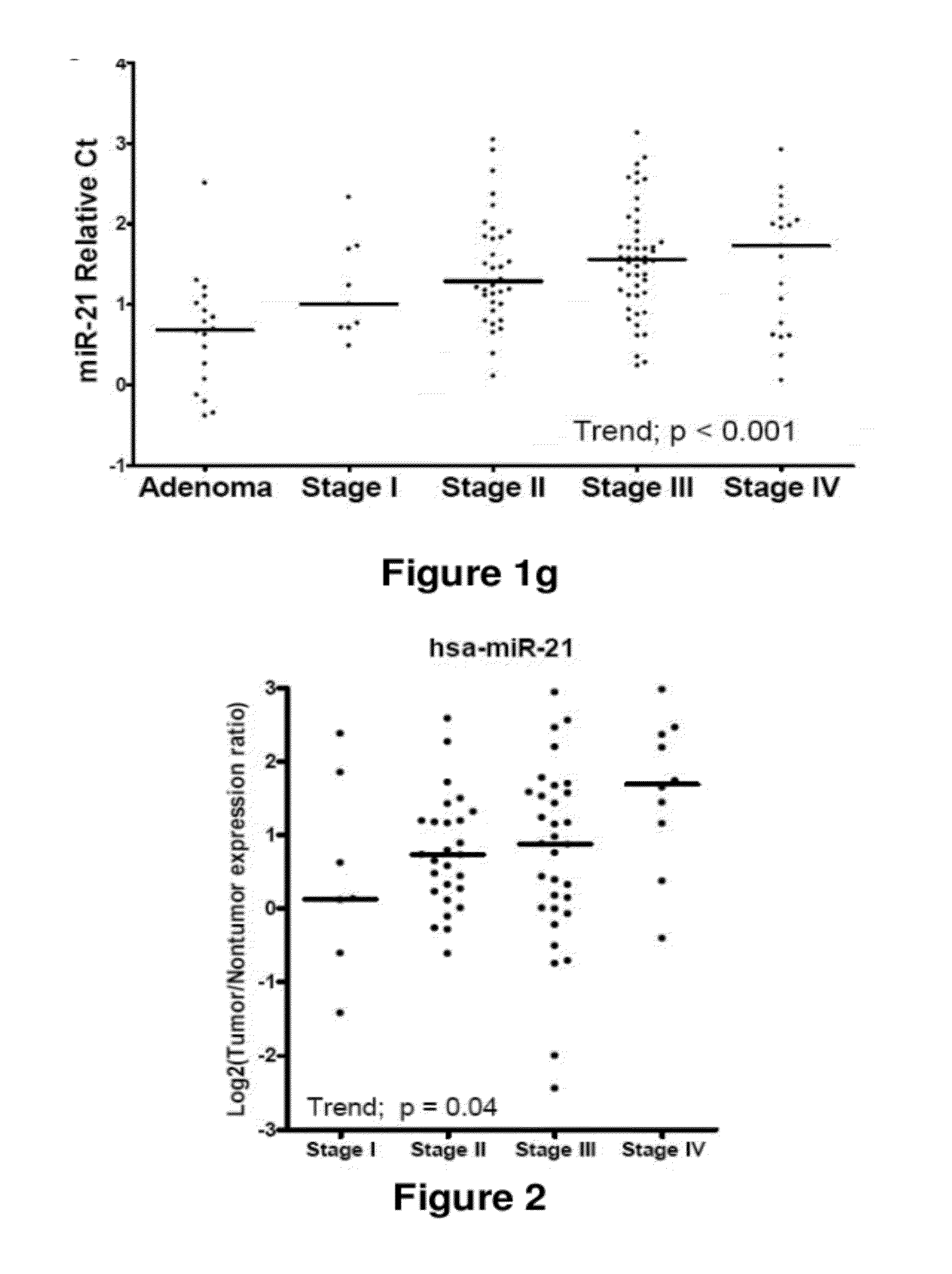
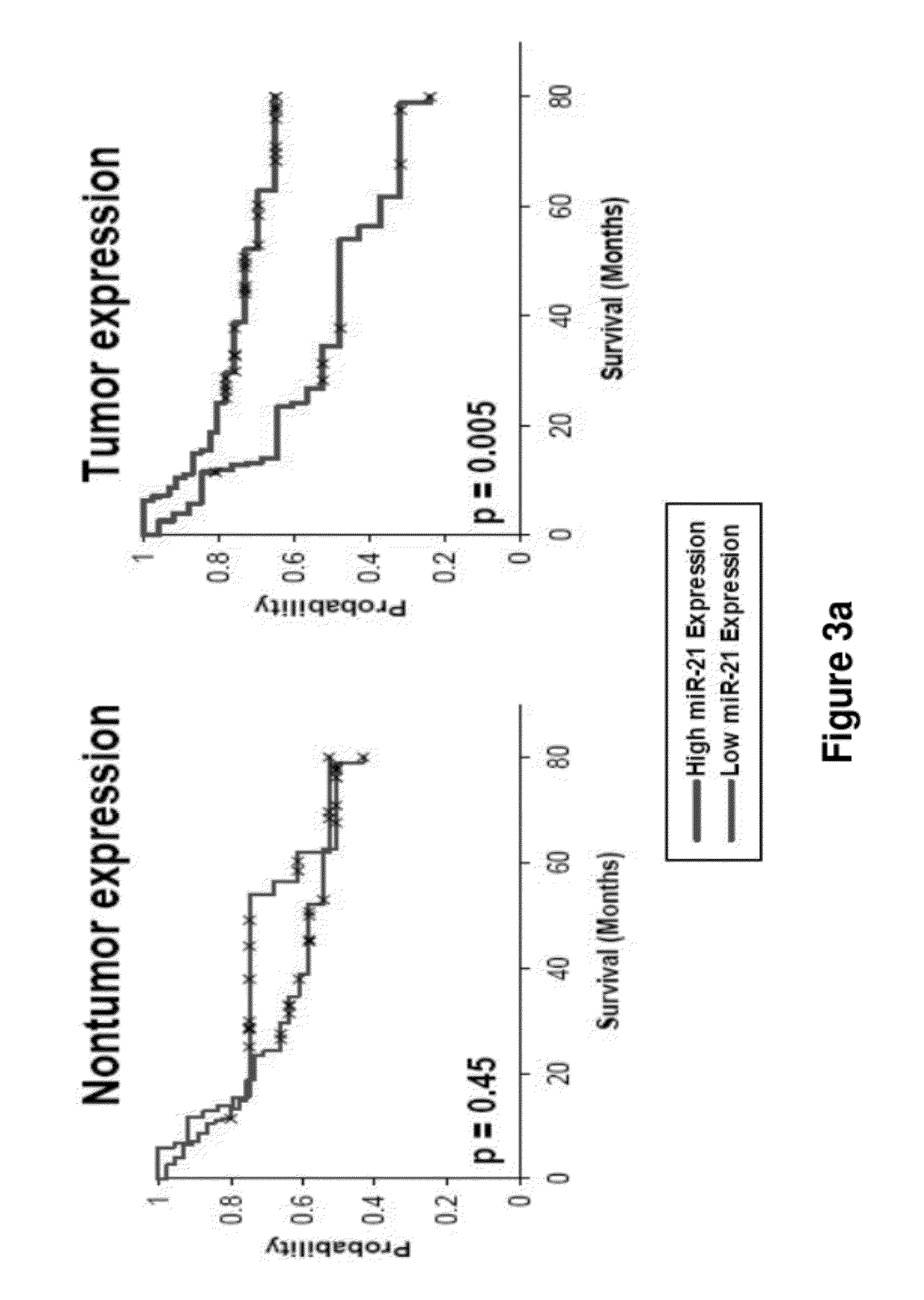
Method of predicting chemotherapy effectiveness in colon adenocarcinoma patients, using mir-21
Owner:THE OHIO STATE UNIV RES FOUND +1
METHOD OF DIAGNOSING POOR SURVIVAL PROGNOSIS COLON CANCER USING miR-10a
InactiveUS20120058915A1Microbiological testing/measurementDigestive systemSurvival prognosisWilms' tumor
The present invention provides novel methods and compositions for the diagnosis and treatment of colon cancers. In particular, the present invention provides diagnostics and prognostics for colon (including colon adenocarcinoma) cancer patients, wherein the methods related to measuring miR levels can predict poor survival. The invention also provides methods of identifying inhibitors of tumorigenesis.
Owner:THE OHIO STATE UNIV RES FOUND +1
METHOD OF DIAGNOSING POOR SURVIVAL PROGNOSIS COLON CANCER USING miR-106a
InactiveUS20120065097A1Microbiological testing/measurementDigestive systemSurvival prognosisMir 106a
The present invention provides novel methods and compositions for the diagnosis and treatment of colon cancers. In particular, the present invention provides diagnostics and prognostics for colon (including colon adenocarcinoma) cancer patients, wherein the methods related to measuring miR levels can predict poor survival. The invention also provides methods of identifying inhibitors of tumorigenesis.
Owner:THE OHIO STATE UNIV RES FOUND +1
METHOD OF DIAGNOSING POOR SURVIVAL PROGNOSIS COLON CANCER USING miR-16b
InactiveUS20120058913A1Decrease survival prognosisMicrobiological testing/measurementDigestive systemSurvival prognosisWilms' tumor
The present invention provides novel methods and compositions for the diagnosis and treatment of colon cancers. In particular, the present invention provides diagnostics and prognostics for colon (including colon adenocarcinoma) cancer patients, wherein the methods related to measuring miR levels can predict poor survival. The invention also provides methods of identifying inhibitors of tumorigenesis.
Owner:THE OHIO STATE UNIV RES FOUND +1
METHOD OF DIAGNOSING POOR SURVIVAL PROGNOSIS COLON CANCER USING miR-203
InactiveUS20120058914A1Microbiological testing/measurementDigestive systemSurvival prognosisWilms' tumor
The present invention provides novel methods and compositions for the diagnosis and treatment of colon cancers. In particular, the present invention provides diagnostics and prognostics for colon (including colon adenocarcinoma) cancer patients, wherein the methods related to measuring miR levels can predict poor survival. The invention also provides methods of identifying inhibitors of tumorigenesis.
Owner:THE OHIO STATE UNIV RES FOUND +1
METHOD OF DIAGNOSING POOR SURVIVAL PROGNOSIS COLON CANCER USING let-7g
InactiveUS20120058912A1Decrease survival prognosisMicrobiological testing/measurementDigestive systemColon adenocarcinomaCancer
The present invention provides novel methods and compositions for the diagnosis and treatment of colon cancers. In particular, the present invention provides diagnostics and prognostics for colon (including colon adenocarcinoma) cancer patients, wherein the methods related to measuring miR levels can predict poor survival. The invention also provides methods of identifying inhibitors of tumorigenesis.
Owner:THE OHIO STATE UNIV RES FOUND +1
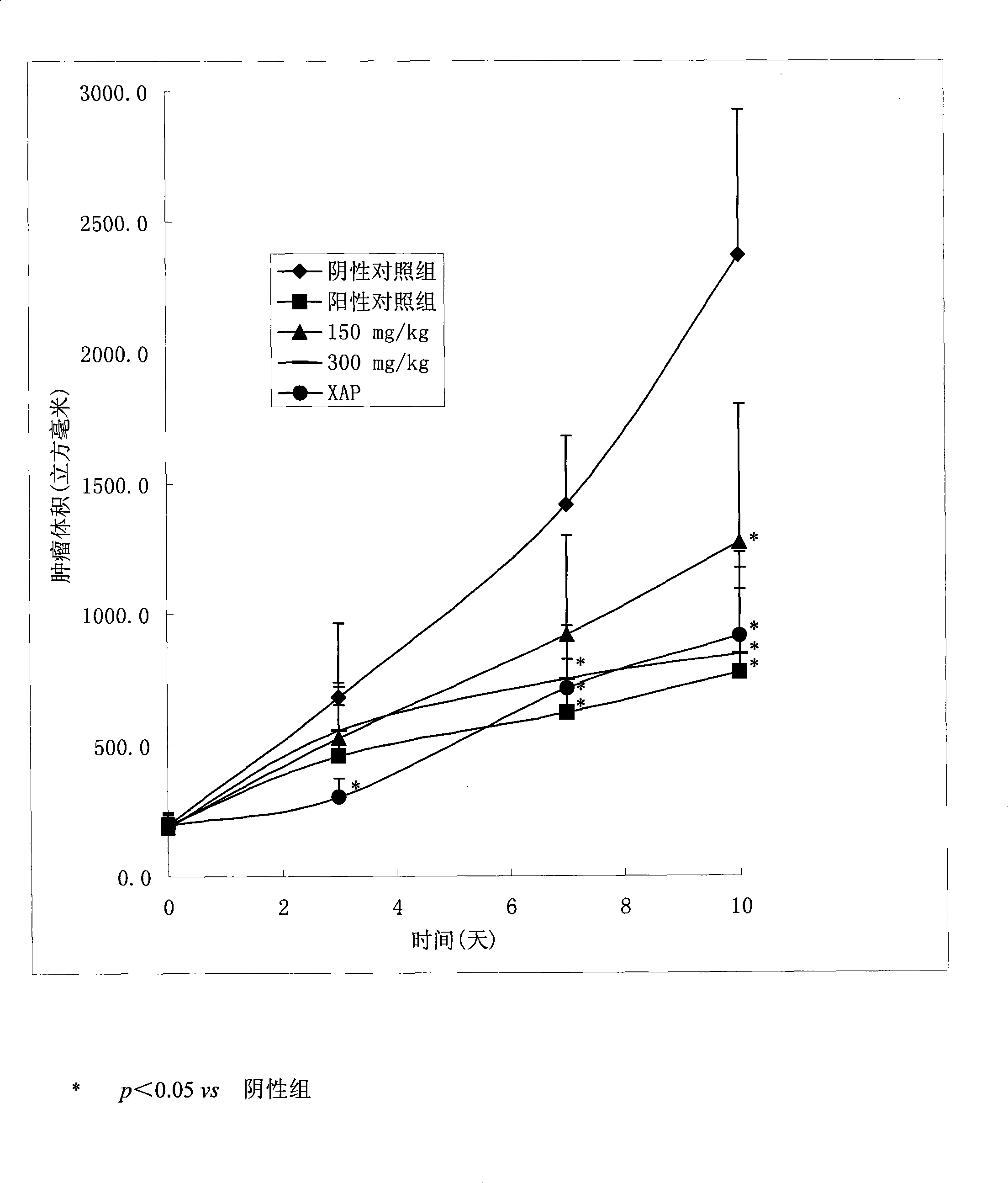
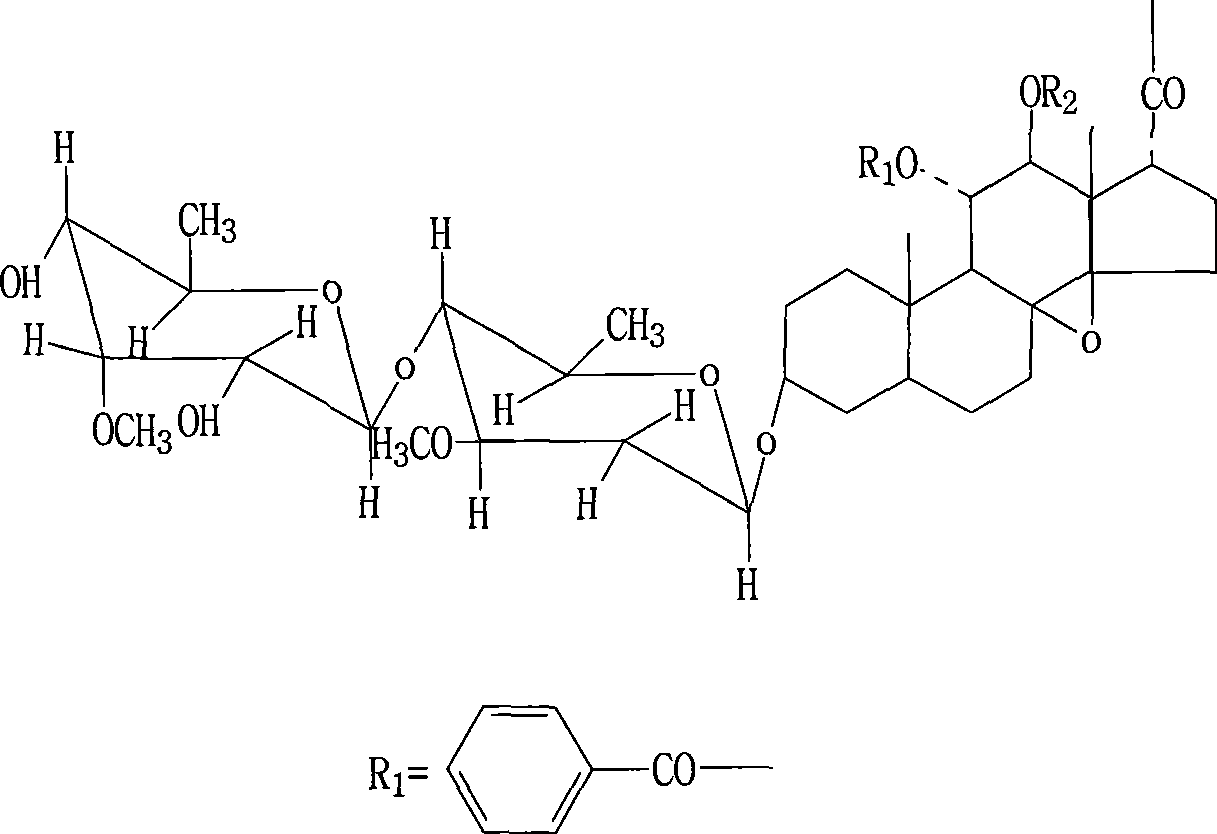
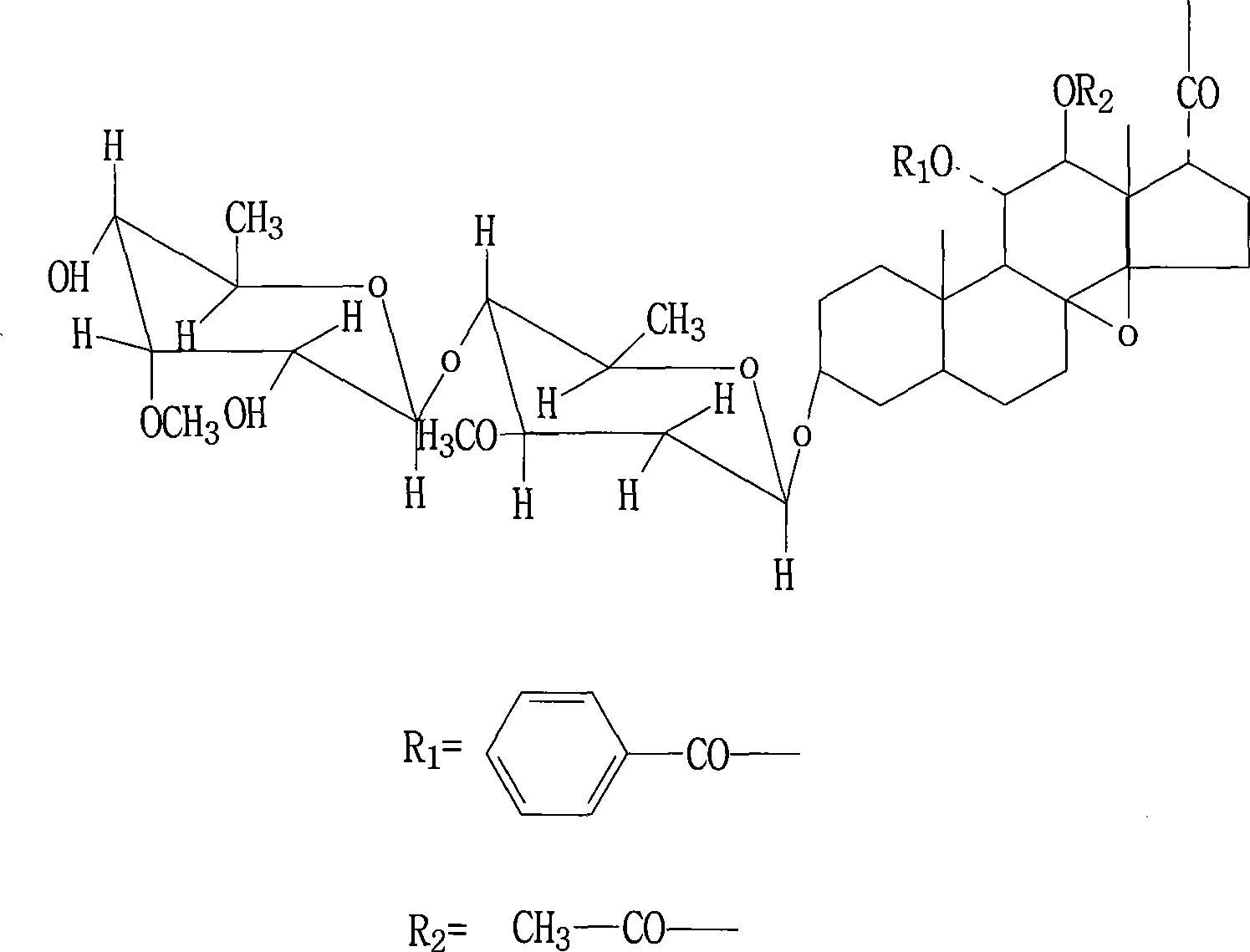
Use of marsdenia tenacissima new glycoside B in preparing medicine for treating tumor disease and preparation method thereof
The invention relates to an application of a compound marsdenia tenacissima glycoside B which is extracted and separated from natural plant marsdenia tenacissima in the preparation of a drug for remedying tumour diseases including various malignant tumour diseases, such as ovary cancer, stomach cancer, liver cancer, leucocythemia, lung cancer, oral epidermoid carcinoma, rectal adenocarcinoma, breast cancer, malignant melanocarcinoma, colon adenocarcinoma, nasopharyngeal carcinoma, prostate cancer, etc. The invention also relates to a preparation technology of the marsdenia tenacissima glycoside B. After using water extraction and alcohol sedimentation, the invention uses an innovative chloroform extraction and aether sedimentation technology by chloroform extraction and also creatively adding aether, thereby reducing the content of impurity in extract obviously, and the marsdenia tenacissima glycoside B with high purity can be obtained just by silica gel column separation at a time. The method has high yield and simple operation, does not require a preparation type precision instrument of high performance liquid phase and other similar instruments and is closer to the requirement of the industrialized production.
Owner:HANGZHOU MINSHENG PHARM CO LTD
METHOD OF DIAGNOSING POOR SURVIVAL PROGNOSIS COLON CANCER USING miR-181b
InactiveUS20120058911A1Decrease survival prognosisMicrobiological testing/measurementDigestive systemSurvival prognosisWilms' tumor
The present invention provides novel methods and compositions for the diagnosis and treatment of colon cancers. In particular, the present invention provides diagnostics and prognostics for colon (including colon adenocarcinoma) cancer patients, wherein the methods related to measuring miR levels can predict poor survival. The invention also provides methods of identifying inhibitors of tumorigenesis.
Owner:THE OHIO STATE UNIV RES FOUND +1
Human ron-related gene variant associated with cancers
ActiveUS20080085510A1TransferasesImmunoglobulins against cell receptors/antigens/surface-determinantsURINARY BLADDER CARCINOMACervix
The invention relates to the nucleic acid and polypeptide sequences of three novel human Ron-related gene variants (Ron-V1, Ron-V2, and Ron-V3). The invention also provides a process for producing the polypeptides of the variants, as well as uses for the nucleic acid, polypeptide and antibodies to same in diagnosing human breast carcinoma, breast adenocarcinoma, cervix epidermoid carcinoma, cervix epitheloid carcinoma, colon adenocarcinoma, urinary bladder carcinoma, prostate carcinoma, esophagus epidermoid carcinoma and esophagus carcinoma.
Owner:VISGENEER
Therapy of p53 mutant colon adenocarcinoma, breast cancer and lung cancer
Owner:PETER MACCALLUM CANCER INST
Genome capture and sequencing for comprehensive chromatin structure maps in complex genomes and cancer progression
ActiveUS10920280B1Efficiently and cost-effectively measuringEfficient and effective early detectionSugar derivativesHydrolasesAdenocarcinoma colonPulmonary adenocarcinoma
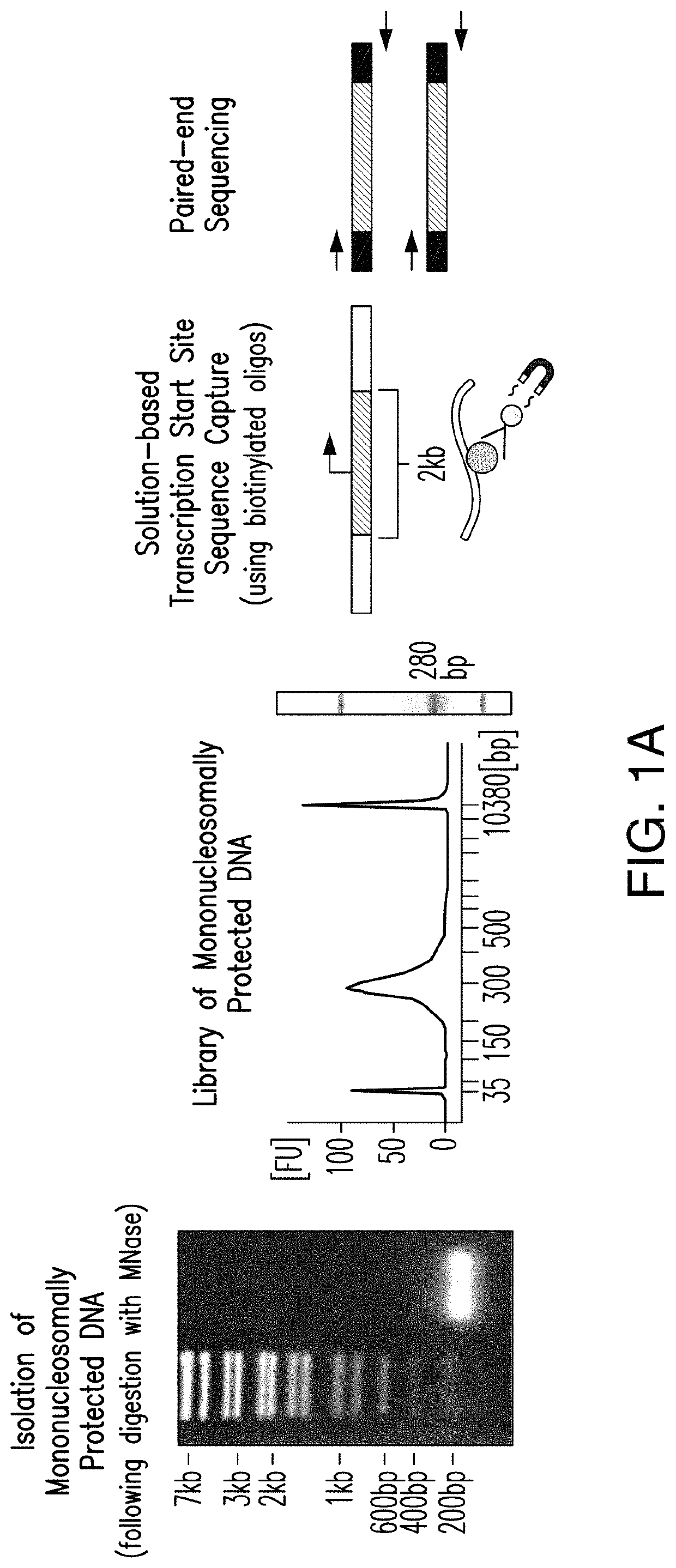
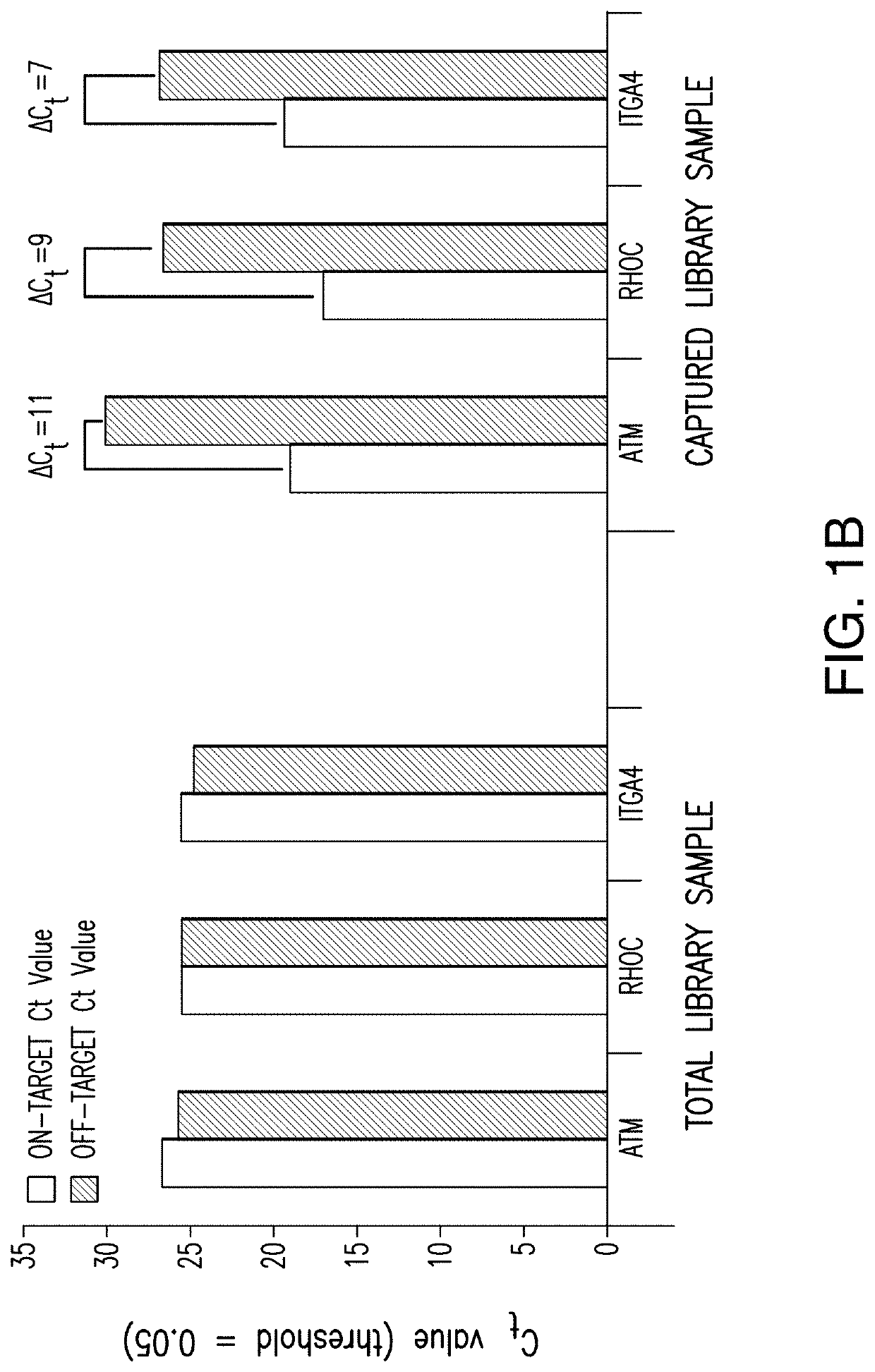
A MNase-Sequence Capture method, mTSS-seq, was developed herein to map genome-wide nucleosome distribution in cancer, for example primary human lung and colon adenocarcinoma tissue. Here, it was confirmed that nucleosome redistribution is an early, widespread event in lung adenocarcinoma (LAC) and colon adenocarcinoma (CRC). These altered nucleosome architectures are consistent between LAC and CRC patient samples indicating that they can serve as important early adenocarcinoma markers. As such, this consistency would be expected in other adenocarcinomas, as well as other carcinomas. It was demonstrated that the nucleosome alterations are driven by the underlying DNA sequence and potentiate transcription factor binding. DNA-directed nucleosome redistributions are widespread early in cancer progression, thus providing a methodology for early detection of cancer in grade one patients.
Owner:FLORIDA STATE UNIV RES FOUND INC
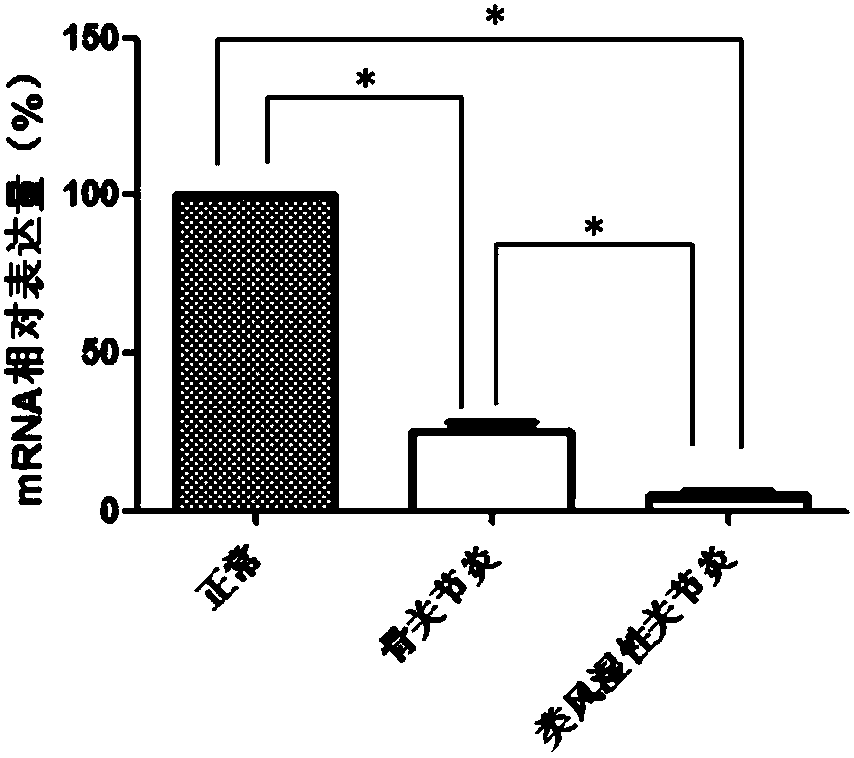
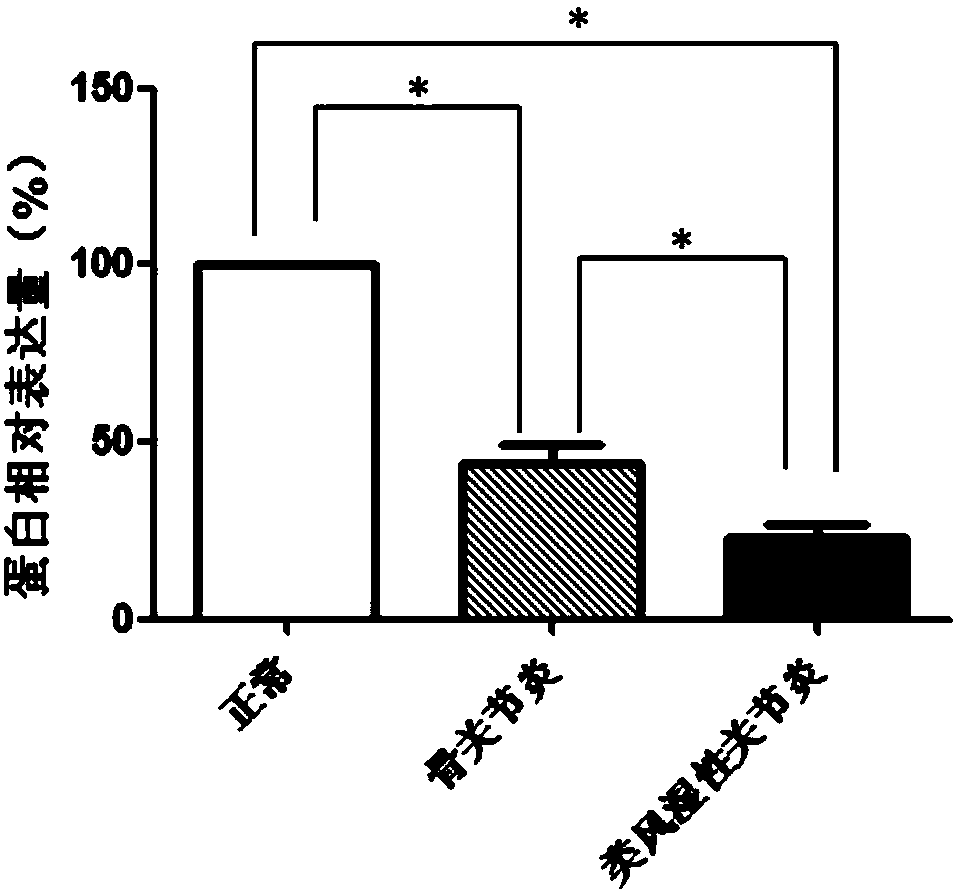
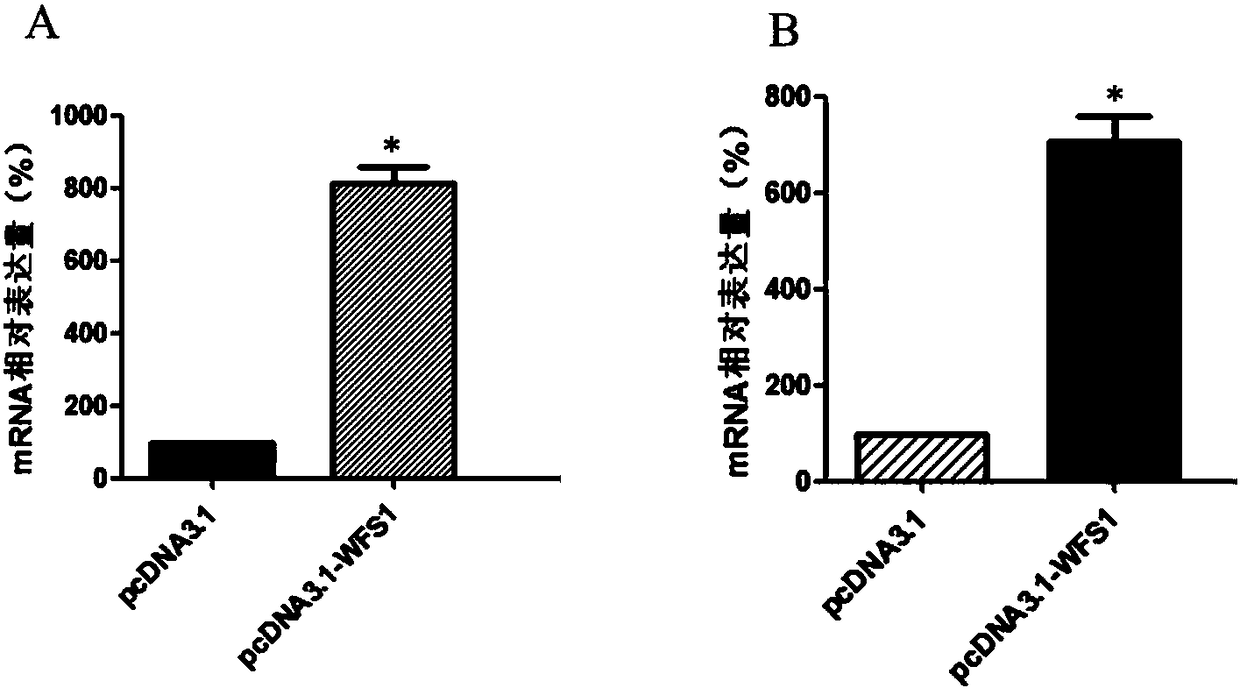
WFS1 (Wolfram 1) as new molecular marker
ActiveCN108486245AExpanded indicationsReduce financial burdenMicrobiological testing/measurementDisease diagnosisMedicineTreatment targets
The invention discloses WFS1 (Wolfram 1) as a treatment target of rheumatoid arthritis and / or osteoarthritis. Hereby, the WFS1 can be used for developing products for diagnosing the rheumatoid arthritis and / or the osteoarthritis and developing drugs for treating the rheumatoid arthritis and / or the osteoarthritis. A research result of the invention provides fundamental basis for a clinicist to makean individual treatment program and is capable of providing a new drug target for development of colon adenocarcinoma drugs.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
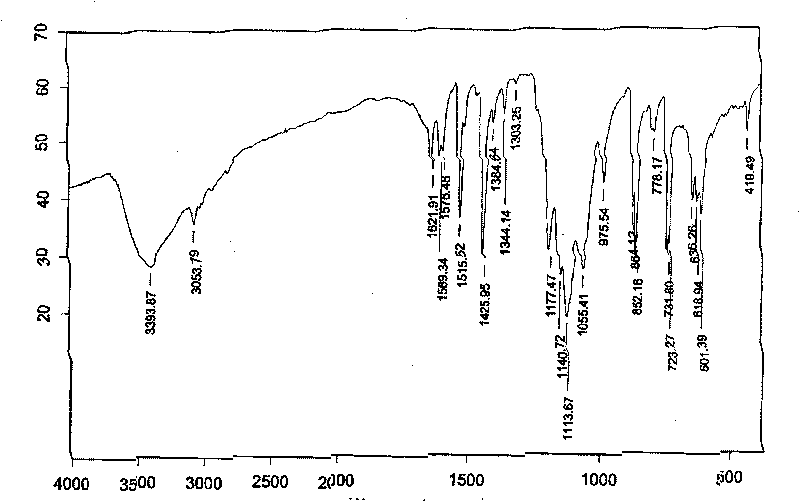
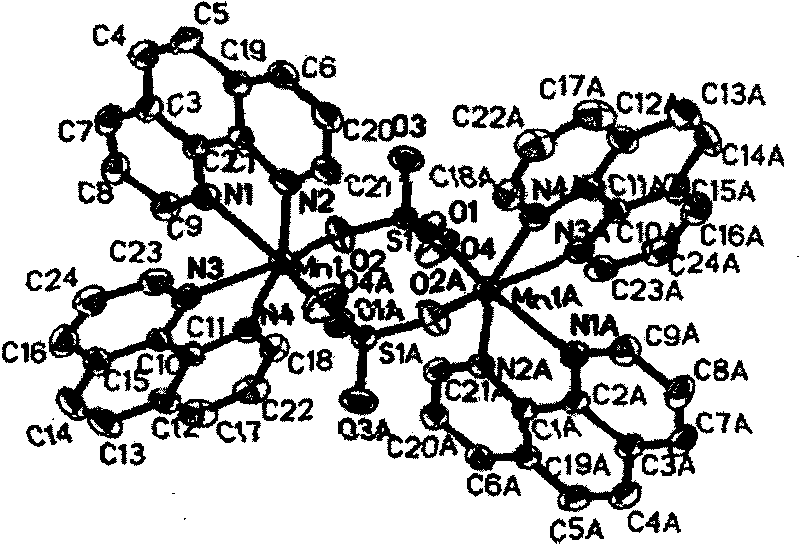
Manganese complex as well as preparation method and uses thereof
InactiveCN101225092BSynthetic raw materials are readily availableLow costOrganic active ingredientsAntineoplastic agentsManganeseNatural state
The invention discloses a manganese complex and the preparation method and application of the manganese complex, providing a complex of manganous, 1, 10-phenanthroline and sulfate. The preparation method is to stir the manganous, 1, 10-phenanthroline and sulfate in the solvent and the complex is acquired after reaction. The preparation method for the manganese complex has the advantages of easy access to the composite material, low cost, precipitation of crystal form with high purity and high yield, stable existence in natural state, good water soluble and fat-soluble performance, excellent inhibition effect to cells of lung cancer, liver cancer, colon adenocarcinoma and a plurality of cancers, wide action spectra and application range.
Owner:CAPITAL NORMAL UNIVERSITY
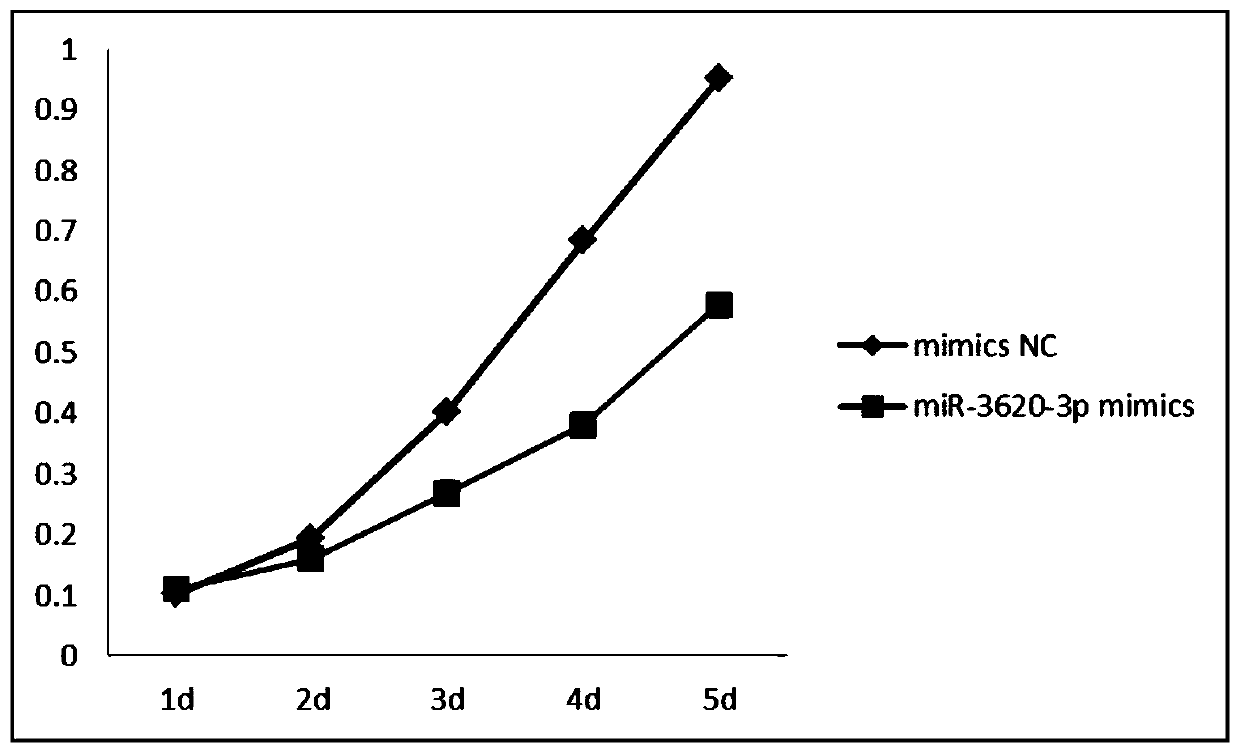
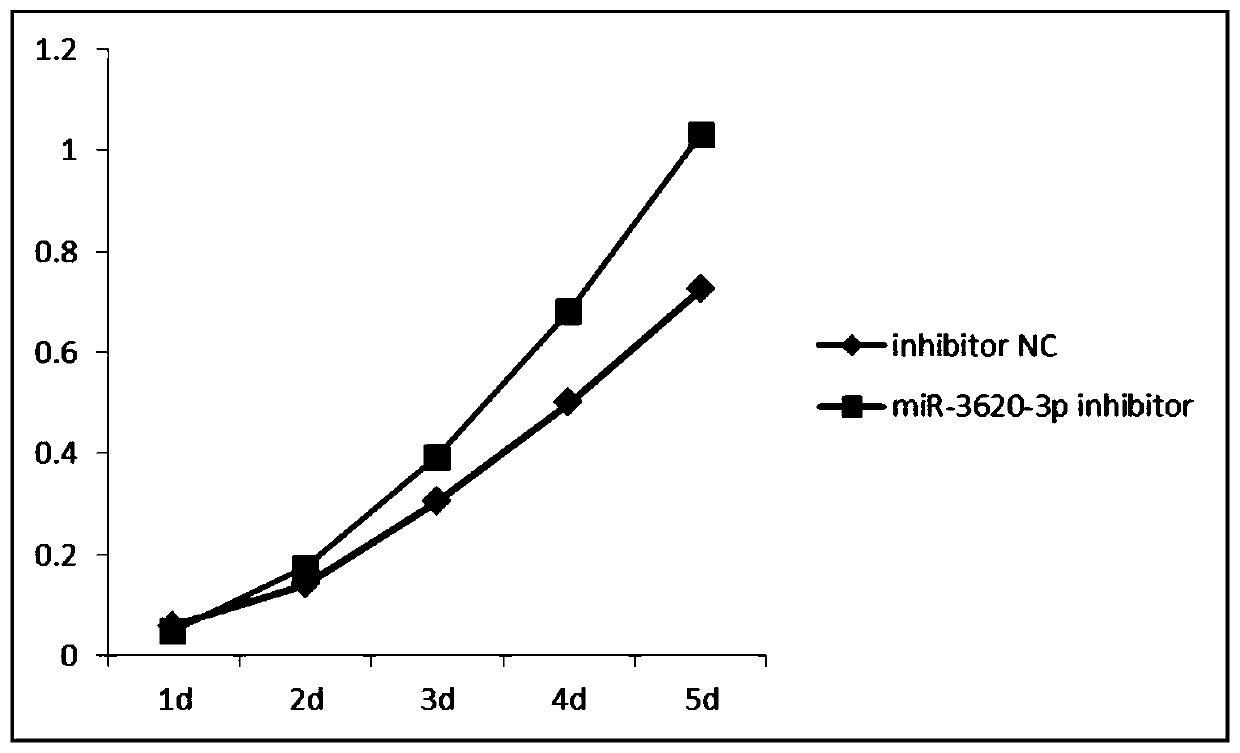
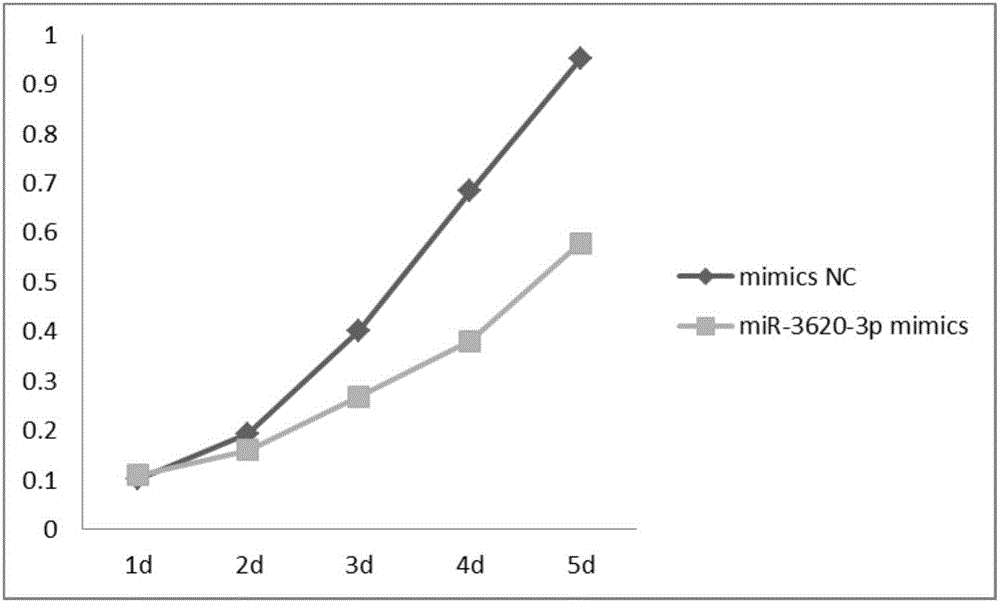
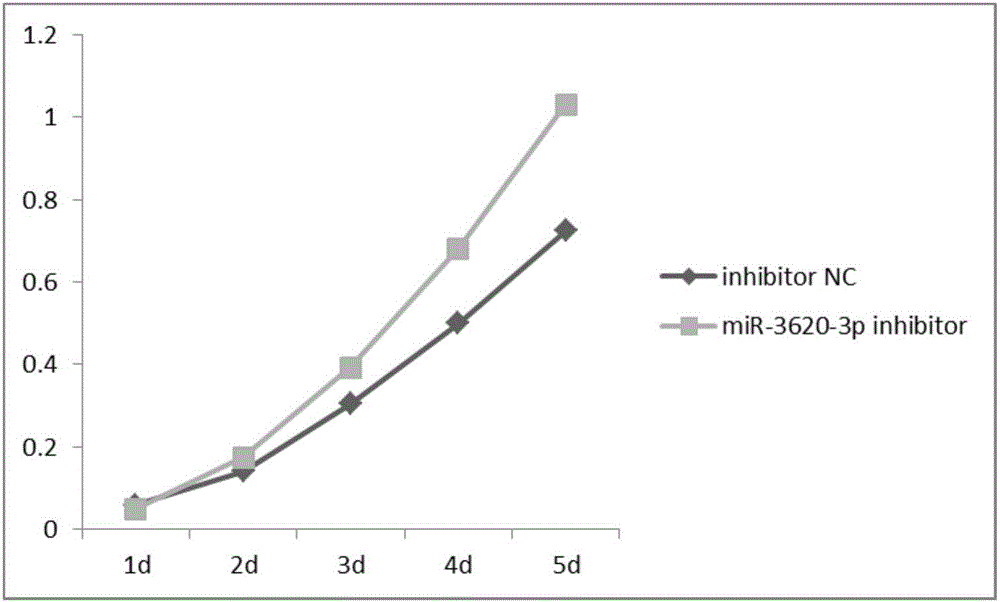
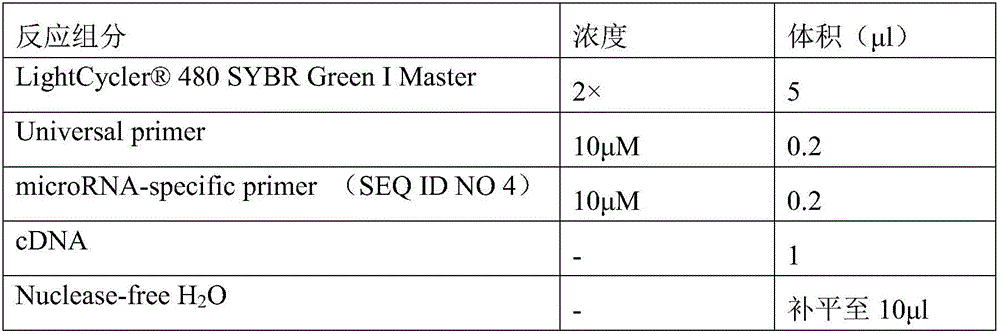
New uses of mir-3620
ActiveCN106244686BOrganic active ingredientsMicrobiological testing/measurementIn vitro proliferationCancer research
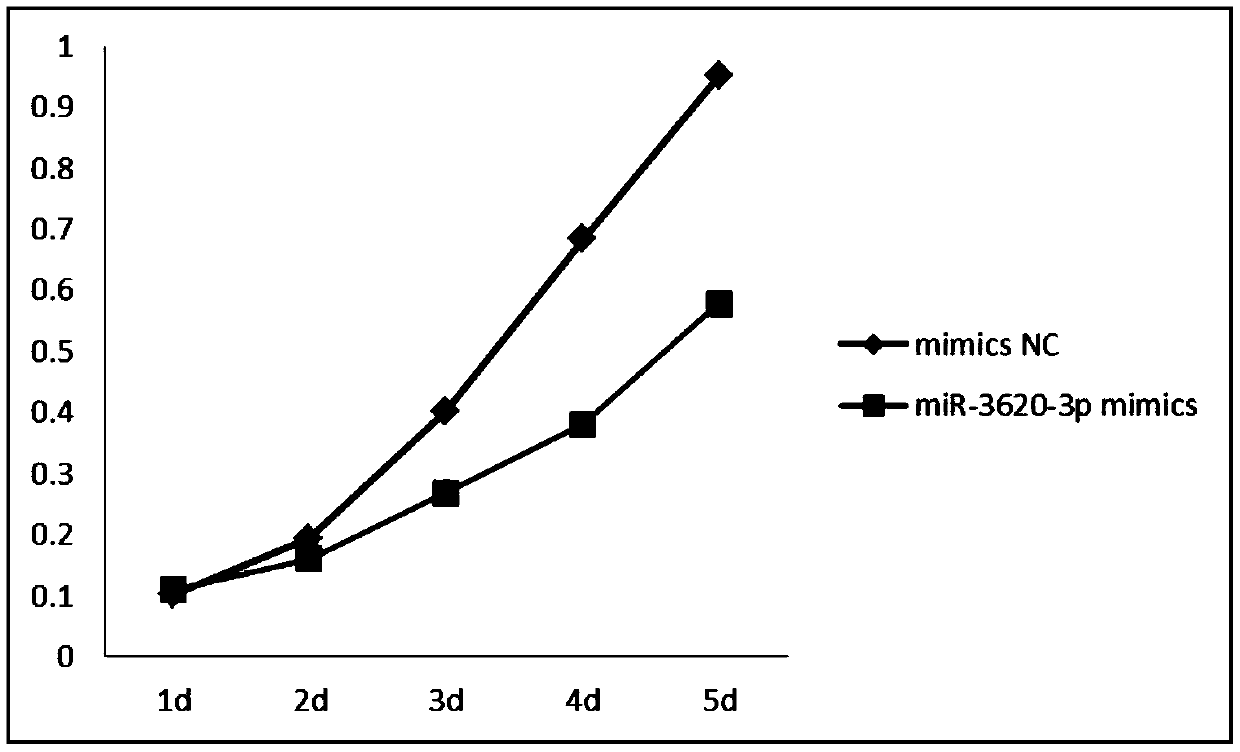
The invention relates to a novel application of mir-3620, in particular to novel applications of the mir-3620 and mature miRNA thereof in diagnosing and treating colon adenocarcinoma. According to the novel application of the mir-3620, next generation sequencing is conducted on colon adenocarcinoma tissues and para-carcinoma tissues, so that data is obtained, and meanwhile, the obtained data is integrated and analyze in combination with existing data of a database, so that miRNA expression data is obtained and candidate mir-3620 is screened out. Further clinical verification and cell experiment results indicate that miR-3620-3p achieves low expression in the cancer tissues, and the over-expression of the miR-3620-3p can inhibit in vitro proliferation capacity of the colon adenocarcinoma cells. The invention provides a novel colon adenocarcinoma diagnosis and treatment gene for clinical application and lays a foundation for further research and development of related reagents.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Modulators of myocyte lipid accumulation and insulin resistance and methods of use thereof
ActiveUS11028061B2Organic active ingredientsOrganic chemistryMyeloid leukemiaHepatocellular carcinoma
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
Modulators of myocyte lipid accumulation and insulin resistance and methods of use thereof
ActiveUS20180222874A1Lowering blood-glucoseIncreasing insulin signalingOrganic active ingredientsOrganic chemistryMyeloid leukemiaHepatocellular carcinoma
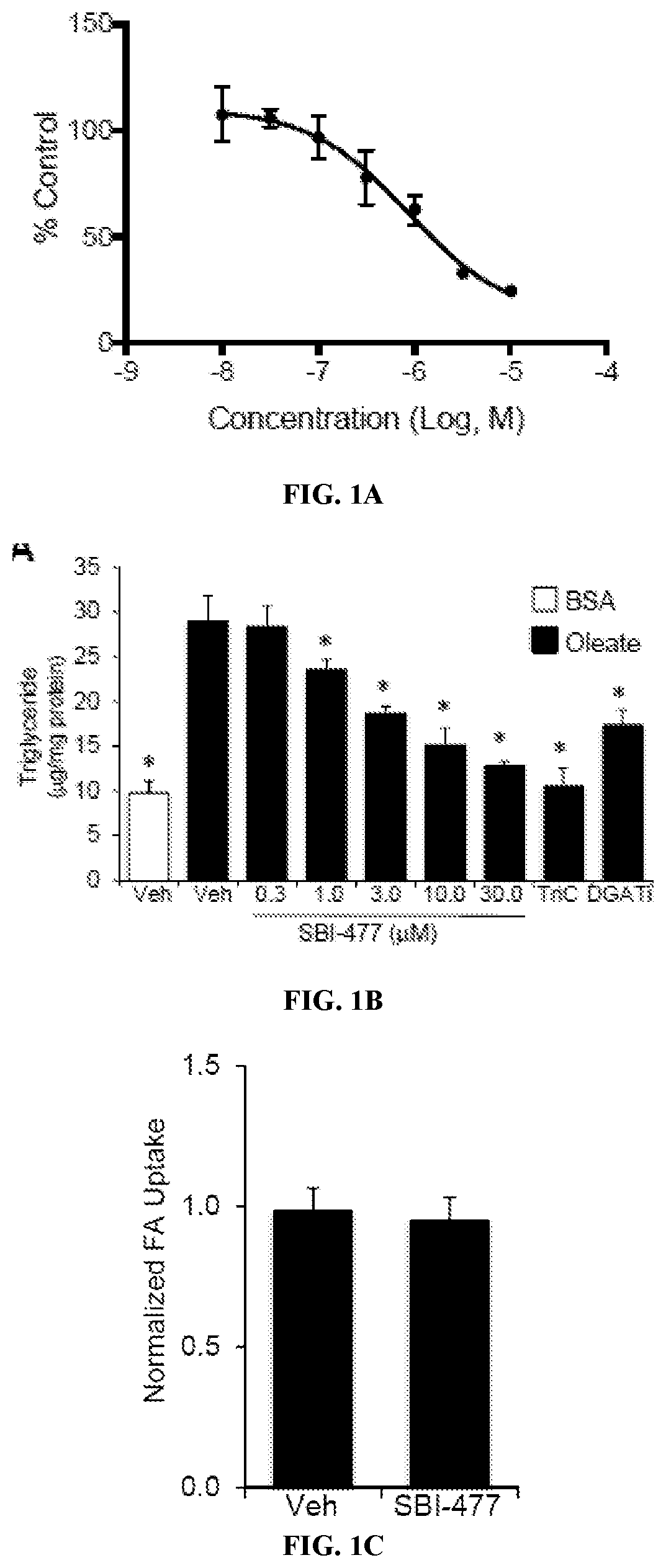
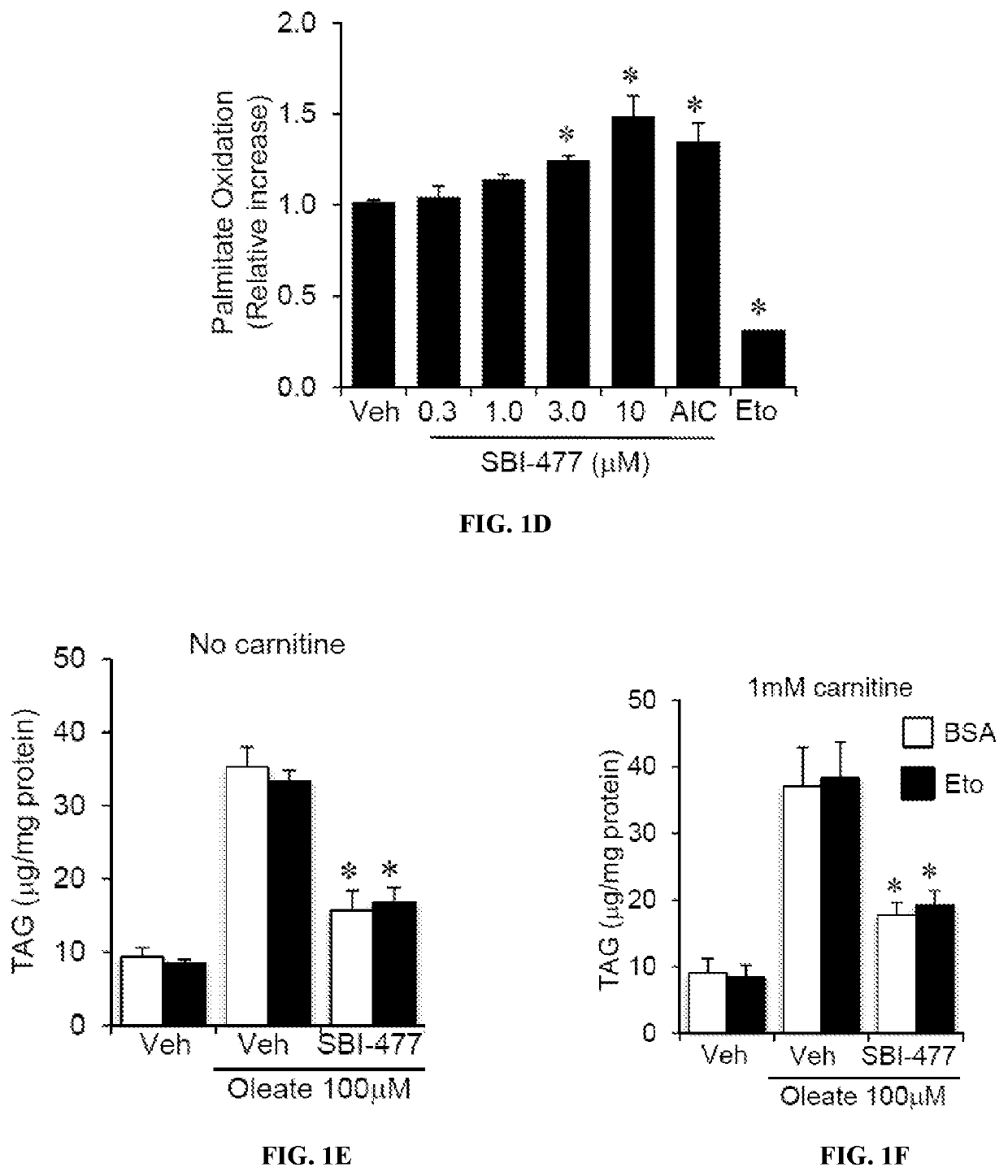
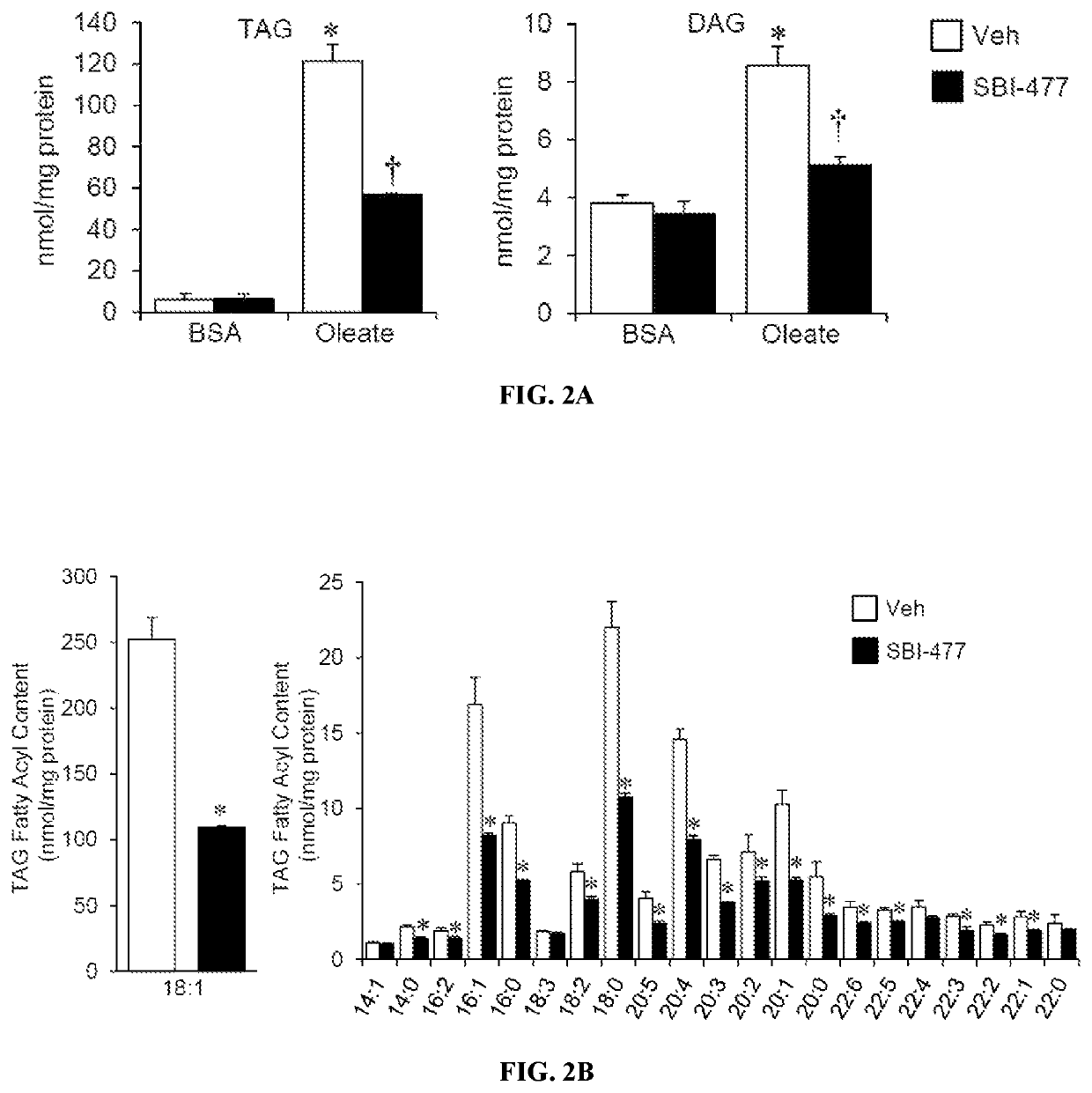
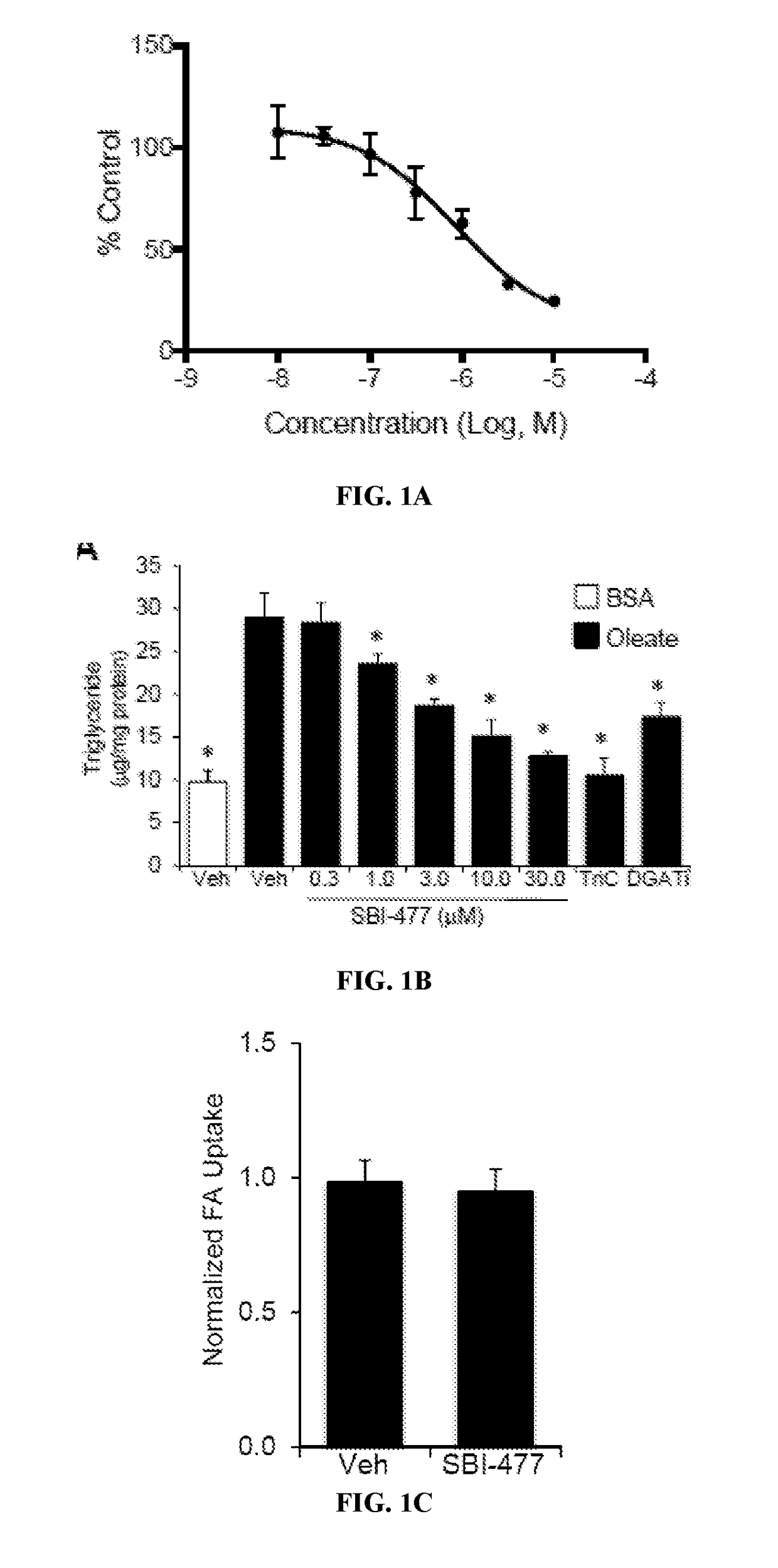
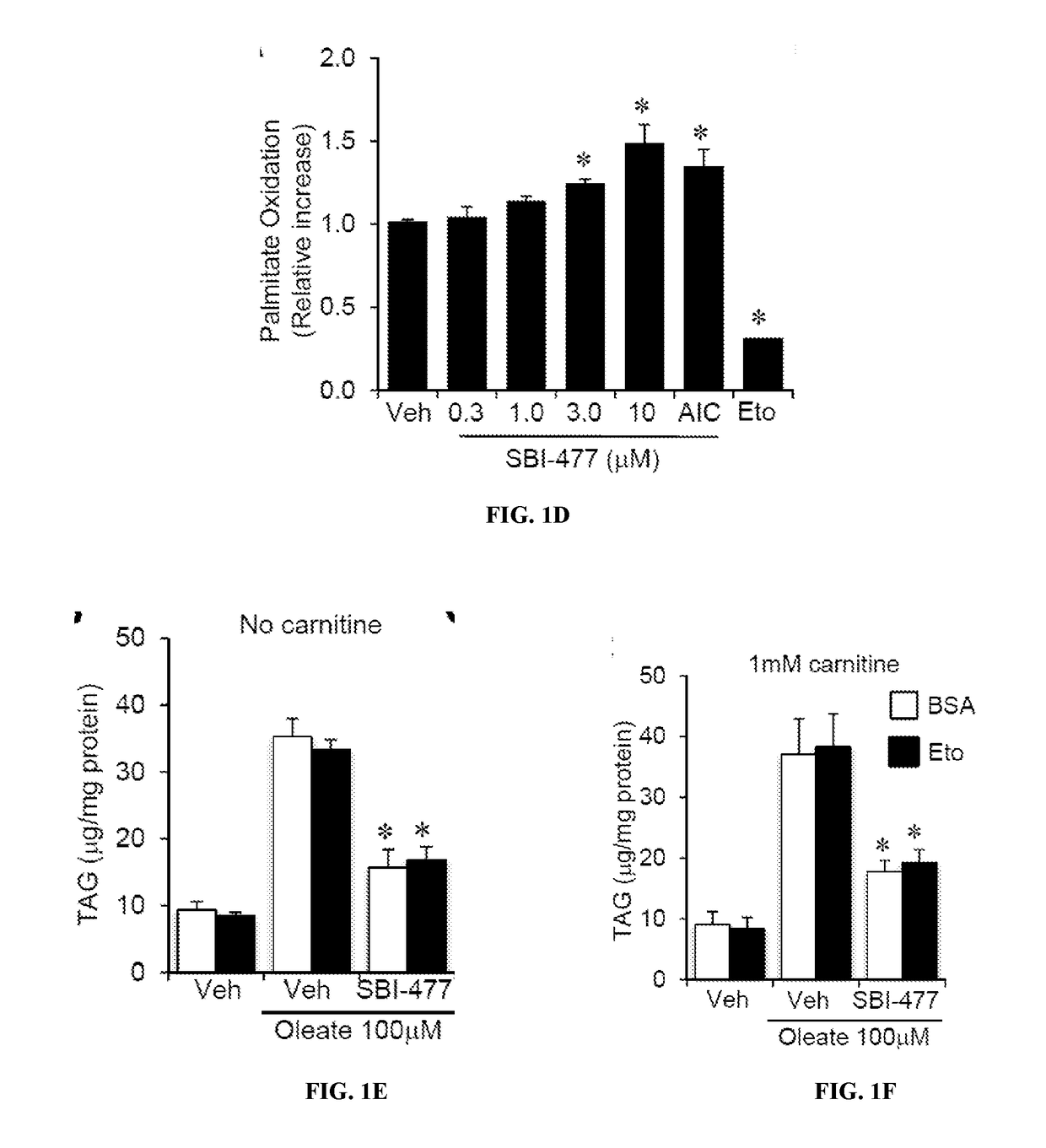
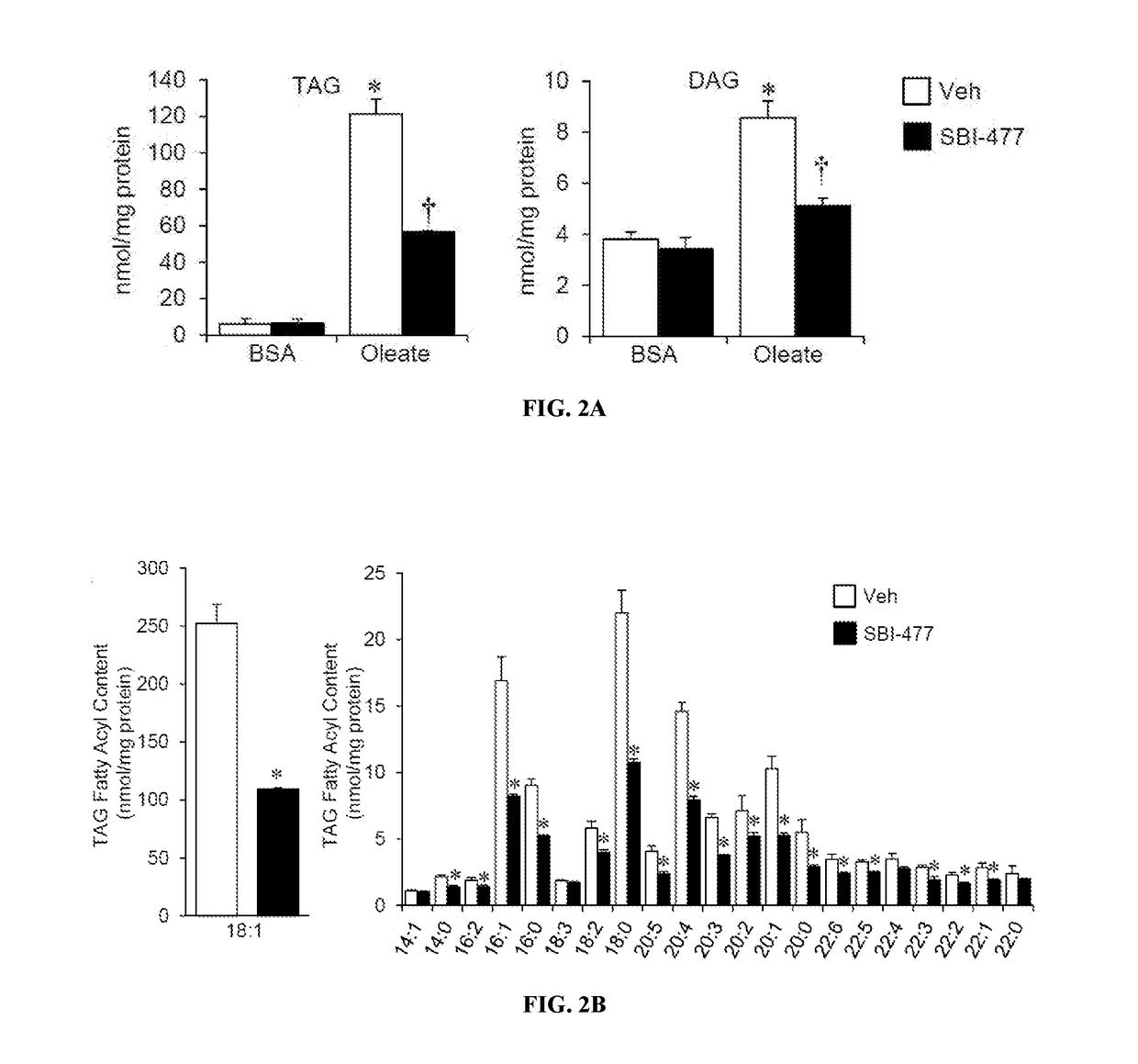
Formulations and methods for reducing blood glucose and / or increasing insulin signaling in a subject have been developed. The formulations include SBI-477 and compounds based on SBI-477 i.e., SBI-477 analogs (collectively, SBI-477 compounds) and / or Mondo family inhibitors, in an effective amount to inhibit intracellular lipid accumulation and / or increase cellular glucose uptake when compared to levels in a control subject not administered the composition. Also disclosed are methods of reducing intracellular lipid accumulation and / or increase glucose uptake in a subject in need thereof. The method includes administering to the subject an effective amount of SBI-477 compounds and / or Mondo family inhibitor to reducing intracellular lipid accumulation and / or increase glucose uptake in the subject. Also disclosed are method for treating one or more Myc-driven cancers, including neuroblastoma, lung squamous cell carcinoma / lung adenocarcinoma, liver hepatocellular carcinoma, colon adenocarcinoma, acute myeloid leukemia, and breast invasive carcinoma.
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
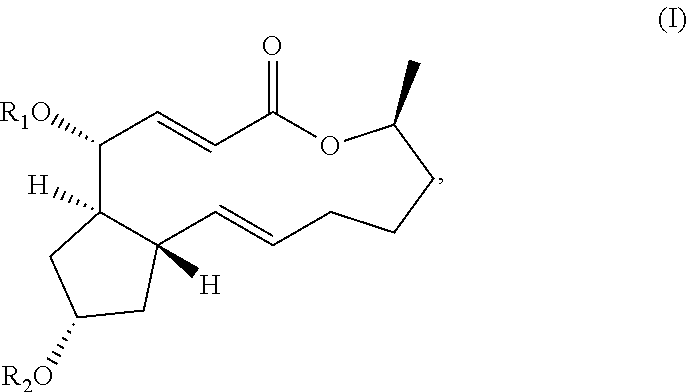
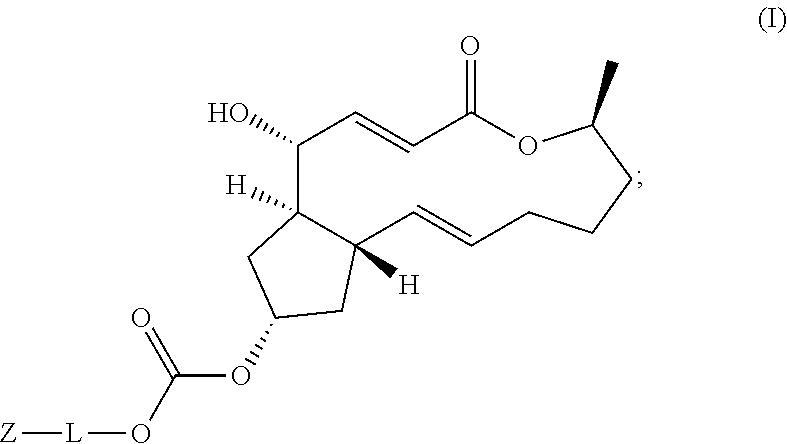
Macrolide brefeldin a ester derivatives and use thereof
PendingUS20220064138A1Prevent proliferationImprove solubilityOrganic active ingredientsOrganic chemistry methodsDiseaseMacrocyclic lactone
The present disclosure relates to the field of medicinal chemistry, and specifically to the use in the inhibition of tumor proliferation activity of ester derivatives of brefeldin A represented by Formula (I). The new compounds provided herein are prominent in the prevention or treatment of hyperproliferative diseases, including liver cancer, leukemia, breast cancer, colon adenocarcinoma, gastric cancer, lung cancer, Bart's esophageal cancer, cervical cancer, pancreatic cancer, endometrial cancer, bone cancer, lymphoma, kidney cancer, brain cancer, nerve cancer, nasopharyngeal cancer, oral cancer and colorectal cancer, and have the potential to be developed as novel antitumor agents.
Owner:OCEAN UNIV OF CHINA
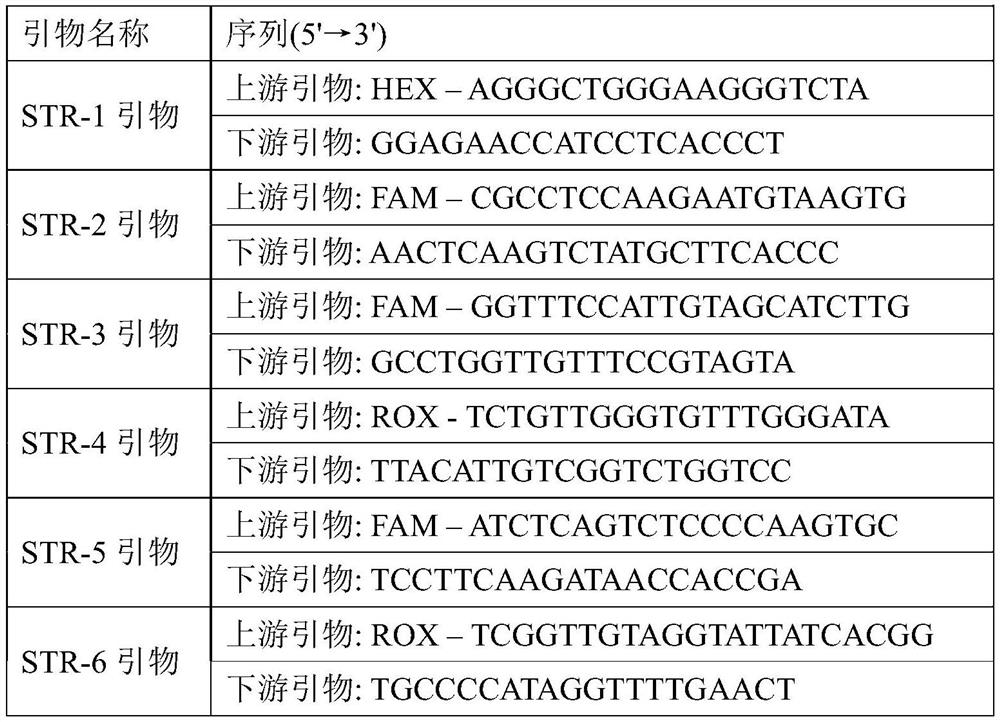
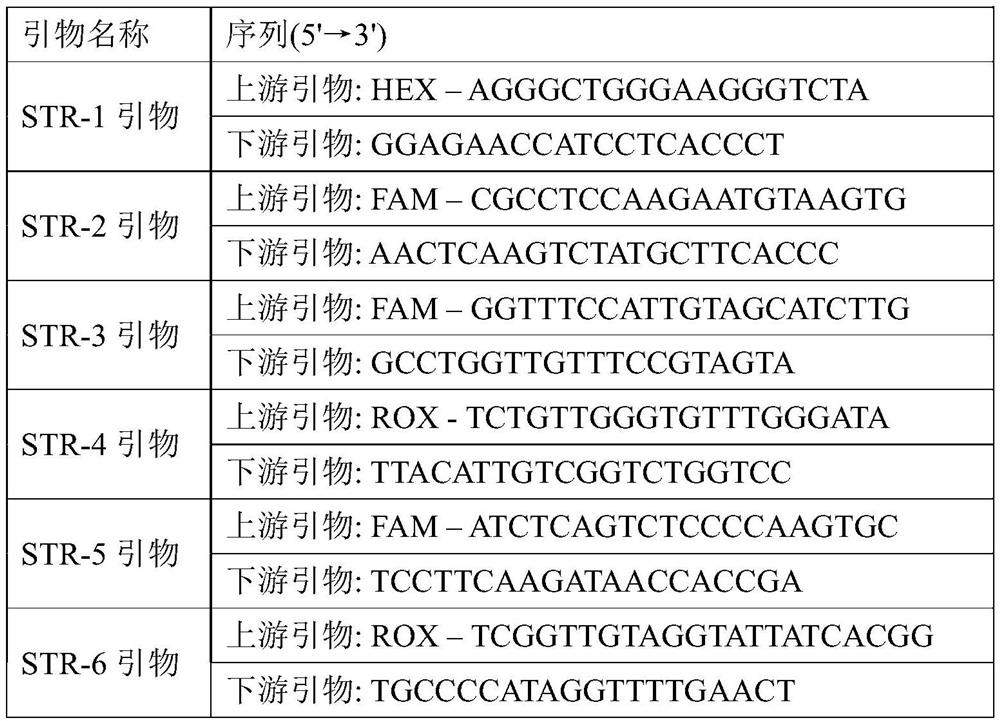
A colon adenocarcinoma susceptibility prediction kit and system
ActiveCN108841960BMicrobiological testing/measurementDNA/RNA fragmentationAdenocarcinoma colonFormamide
The present invention relates to a colon adenocarcinoma susceptibility prediction kit, which comprises the following components: STR-1 primer, STR-2 primer, STR-3 primer, STR-4 primer, STR-5 primer, STR-6 primer; Further, it may also include: PCR amplification reaction solution, LIZ-500 molecular weight internal standard, deionized formamide. The colon adenocarcinoma susceptibility prediction kit of the invention can be used for the diagnosis and susceptibility prediction of colon adenocarcinoma. The invention also provides a colon adenocarcinoma susceptibility prediction system.
Owner:JILIN UNIV
Novel application of mir-3620
ActiveCN106244686AOrganic active ingredientsMicrobiological testing/measurementIn vitro proliferationCancer research
The invention relates to a novel application of mir-3620, in particular to novel applications of the mir-3620 and mature miRNA thereof in diagnosing and treating colon adenocarcinoma. According to the novel application of the mir-3620, next generation sequencing is conducted on colon adenocarcinoma tissues and para-carcinoma tissues, so that data is obtained, and meanwhile, the obtained data is integrated and analyze in combination with existing data of a database, so that miRNA expression data is obtained and candidate mir-3620 is screened out. Further clinical verification and cell experiment results indicate that miR-3620-3p achieves low expression in the cancer tissues, and the over-expression of the miR-3620-3p can inhibit in vitro proliferation capacity of the colon adenocarcinoma cells. The invention provides a novel colon adenocarcinoma diagnosis and treatment gene for clinical application and lays a foundation for further research and development of related reagents.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Prognostic marker and prognostic risk assessment model for metastatic colon adenocarcinoma and application of prognostic marker and prognostic risk assessment model
ActiveCN112143809AGood discernmentImprove expression levelHealth-index calculationMicrobiological testing/measurementColon adenocarcinomaColonic adenocarcinoma
The invention provides a prognostic marker and prognostic risk assessment model for metastatic colon adenocarcinoma and application of the prognostic marker and the prognostic risk assessment model. The prognostic marker comprises a combination of any one or at least two of prognostic related genes LEP, DLX2, CLSTN2 or REG3A. Meanwhile, the invention further provides three potential treatment drugs for the metastatic colon adenocarcinoma, namely ajmaline, TTNPB and dydrogesterone. The prognostic marker provided by the invention is remarkably related with the survival of a colon adenocarcinoma(COAD) patient, and the risk assessment model constructed by utilizing the prognostic marker can carry out risk assessment and survival analysis on patients, and the accuracy is relatively high.
Owner:杭州百可生物科技有限公司
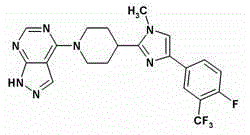
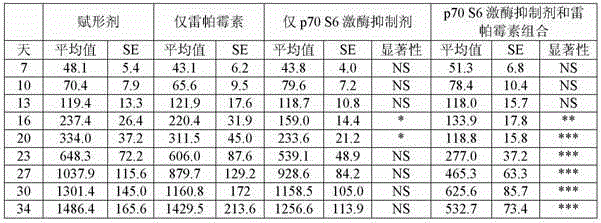
P70 S6 kinase inhibitor and MTOR inhibitor combination therapy
The present invention provides a compound 4-[4-[4-(4-fluoro-3-trifluoromethyl-phenyl)-1-methyl-1H-imidazol-2-yl]-piperidine-1- combination therapy of ]-1H-pyrazolo[3,4-d]pyrimidine or a pharmaceutically acceptable salt thereof and an mTOR inhibitor for the treatment of glioblastoma multiforme, colon adenocarcinoma, non-small cell lung cancer , small cell lung cancer, cisplatin-resistant small cell lung cancer, ovarian cancer, leukemia, pancreatic cancer, prostate cancer, breast cancer, renal cell carcinoma, multiple myeloma, Kaposi's sarcoma, Hodgkin's lymphoma, Lymphangioleiomyoma, non-Hodgkin lymphoma, or sarcoma.
Owner:ELI LILLY & CO
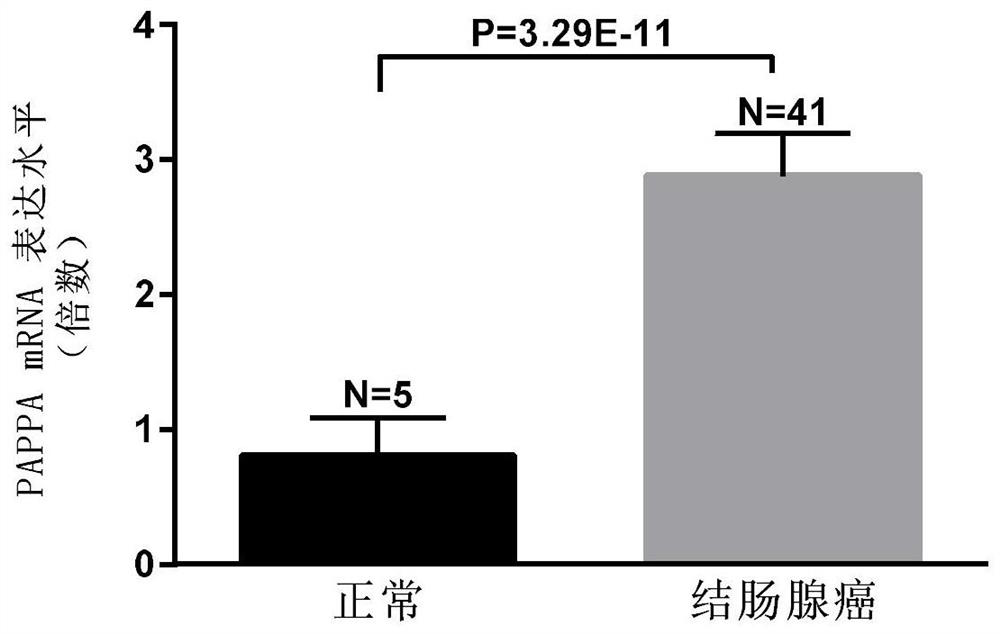
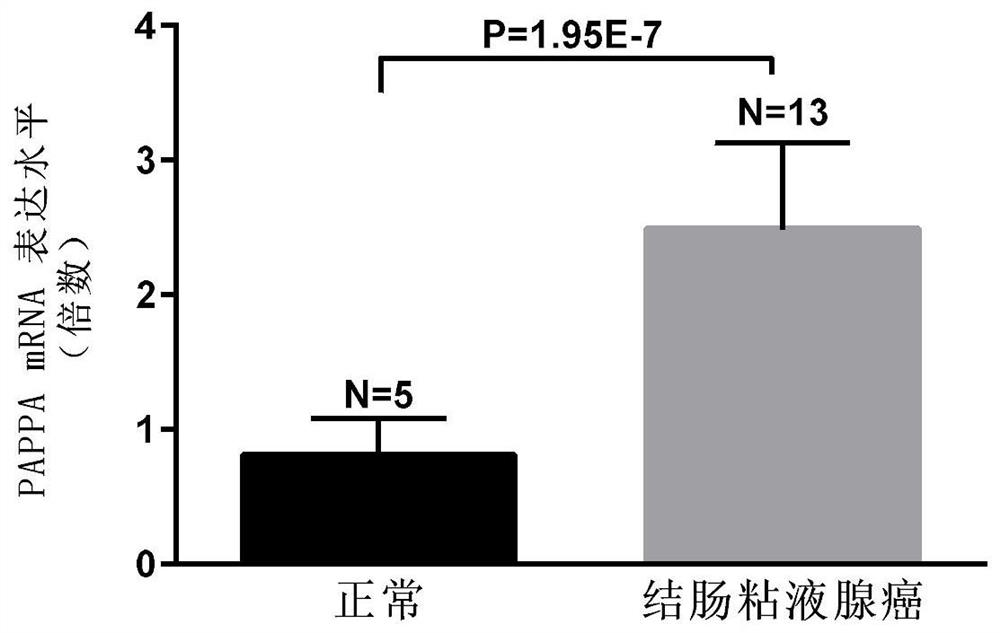
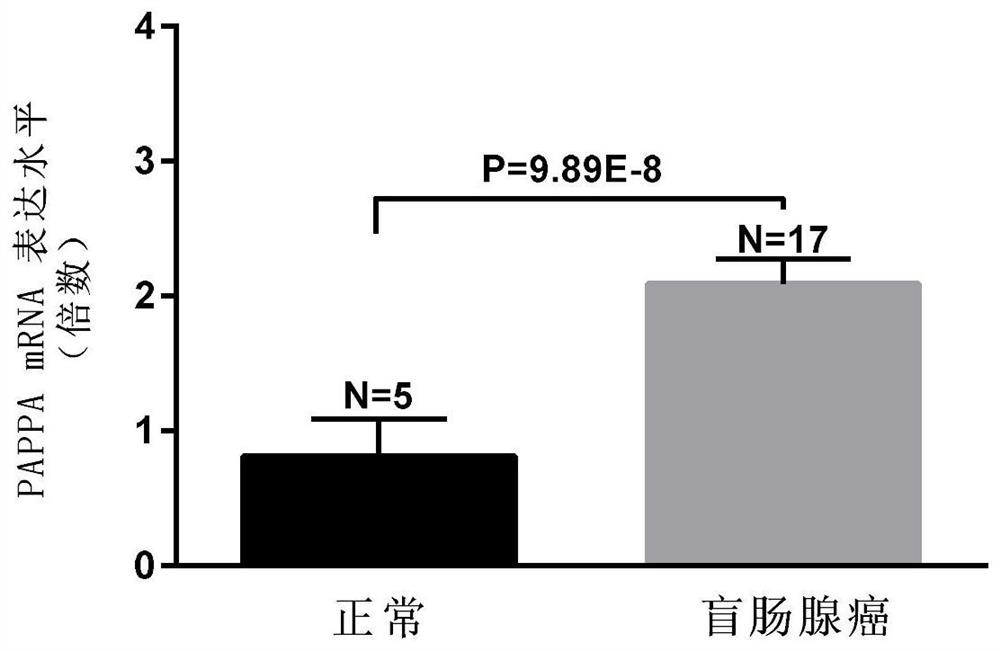
Biomarkers for the detection of colorectal cancer and their applications
ActiveCN108676893BEasy to operateStrong specificityMicrobiological testing/measurementMaterial analysisColon sigmoideumBlood plasma
The invention relates to a diagnostic marker, and specifically provides a biomarker for detecting colorectal cancer and its application. The present invention finds through experimental research that the mRNA expression level of pregnancy-associated plasma protein A (PAPPA) gene in colon adenocarcinoma tissue, colonic mucinous adenocarcinoma tissue, cecum adenocarcinoma tissue, sigmoid colorectal cancer tissue and rectal adenocarcinoma tissue and healthy control There were significant differences between them; the expression level of PAPPA protein in the serum of patients with colorectal cancer was also significantly higher than that of normal controls. The present invention proposes the correlation between PAPPA and colorectal cancer for the first time, and verifies through experiments that the available PAPPA can be used to diagnose colorectal cancer, and has the characteristics of simple operation, high specificity and high sensitivity.
Owner:江西佰延生物技术有限公司
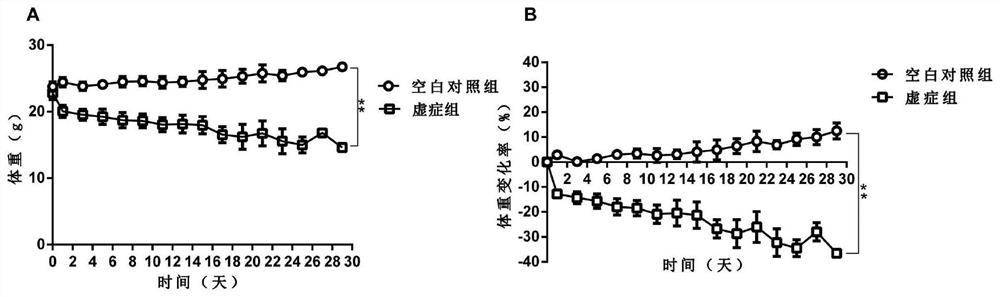
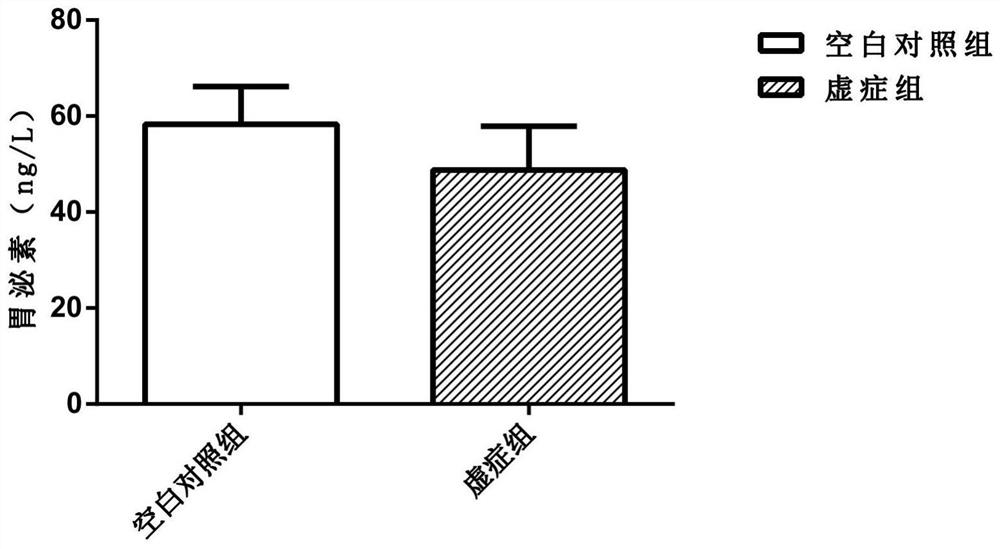
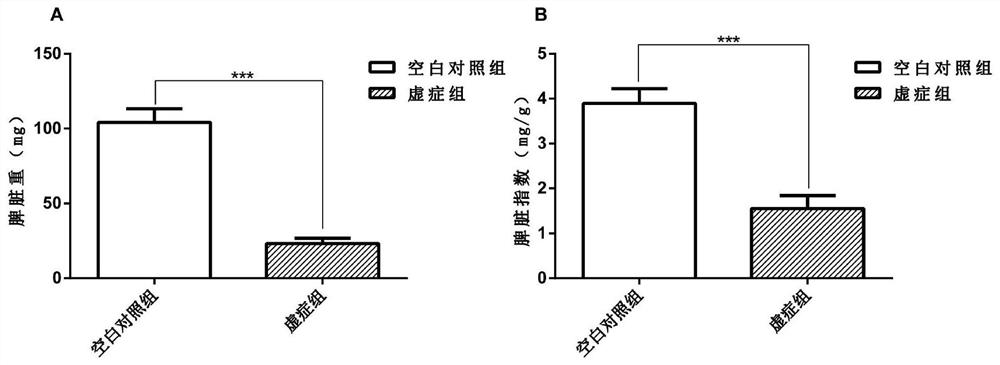
A Method for Establishing a Deficiency Syndrome Tumor Cachexia Model
ActiveCN109528815BCompounds screening/testingMammal material medical ingredientsColonic adenocarcinomaDisease
The invention provides a method for establishing a tumor cachexia model of deficiency syndrome. The method comprises, at the same time, adopting the three-factor induction method of bitter cold diarrhea, hunger and fullness, and exhausted exercise to establish a deficiency syndrome mouse model, and inducing it for 7-15 days; inoculating the obtained deficiency syndrome mice with C26 colon adenocarcinoma cells to obtain deficiency syndrome Tumor cachexia model: After inoculation, the two-factor induction method of bitter cold diarrhea and abnormal hunger and fullness is adopted at the same time, and the induction is continued for 15‑18 days. Among them, body weight, thymus weight, spleen weight, and gastrin content were selected as observation indicators for deficiency syndrome. The animal model established by the above method can be used to study deficiency syndrome tumor cachexia or to screen drugs for treating deficiency syndrome tumor cachexia.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
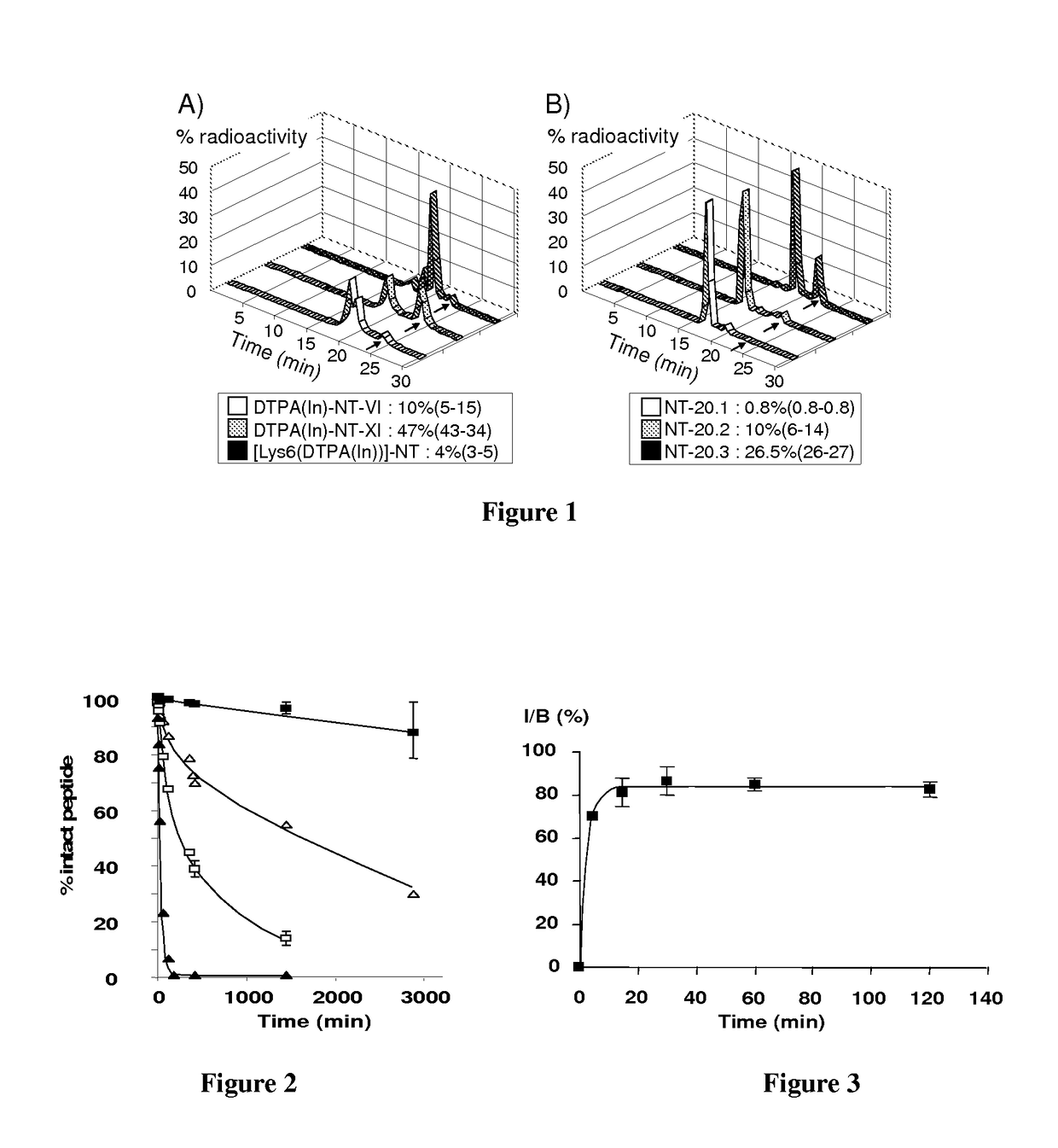
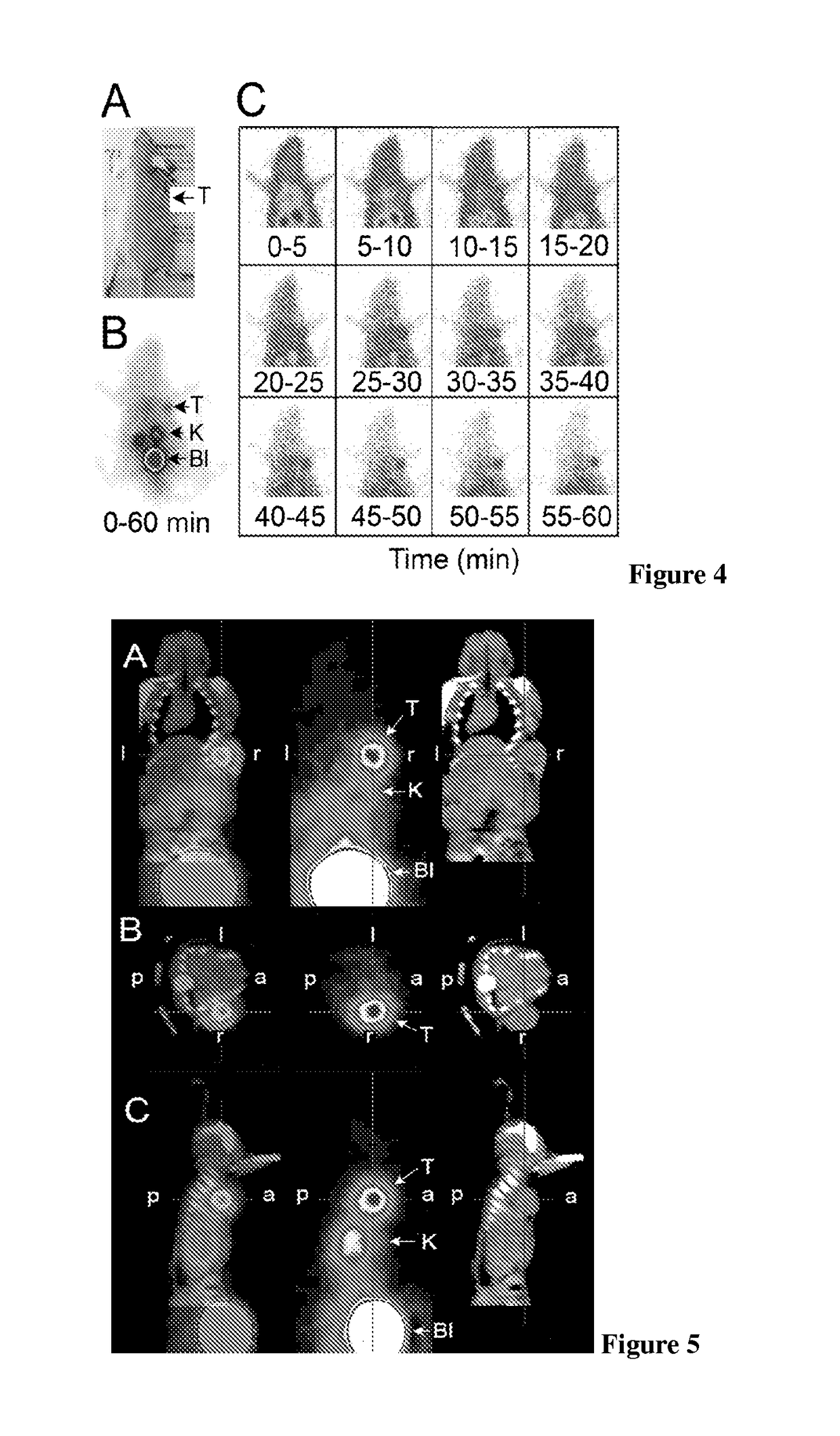
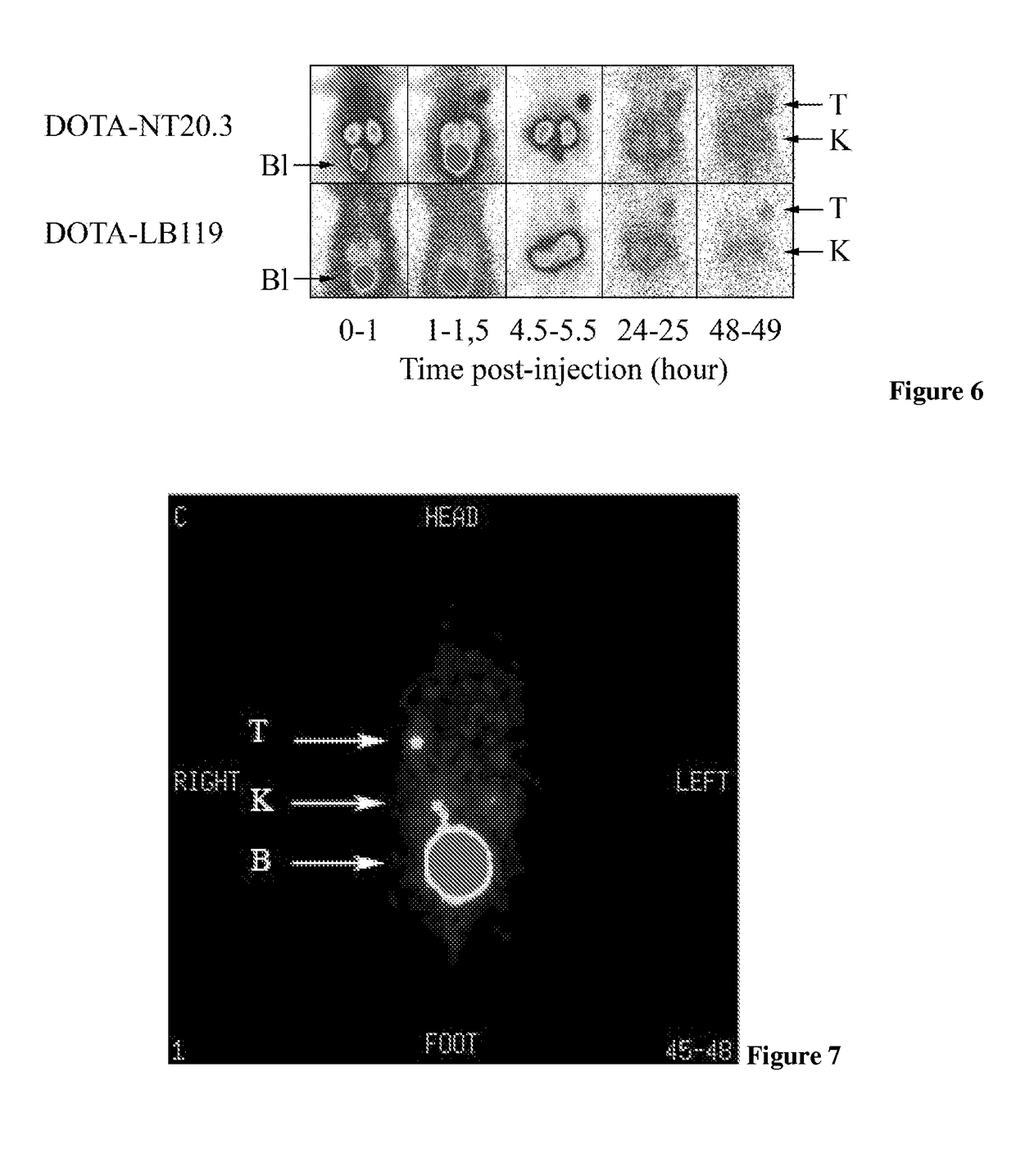
Neurotensin analogues for radioisotope targeting to neurotensin receptor-positive tumors
ActiveUS9809624B2Prevent relapseArresting its developmentPeptide/protein ingredientsPeptide sourcesSmall-cell carcinomaEwing's sarcoma
The invention relates to a new neurotensin analogue, or a salt thereof, useful for targeting to neurotensin receptor-positive tumors, like ductal pancreatic adenocarcinoma, exocrine pancreatic cancer, invasive ductal breast cancers, colon adenocarcinoma, small cell lung carcinoma, Ewing sarcoma, meningioma, medulloblastoma and astrocytoma.
Owner:IASON GMBH
Human Ron-related gene variant associated with cancers
ActiveUS7452985B2Sugar derivativesMicrobiological testing/measurementURINARY BLADDER CARCINOMAAntiendomysial antibodies
The invention relates to the nucleic acid and polypeptide sequences of three novel human Ron-related gene variants (Ron-V1, Ron-V2, and Ron-V3). The invention also provides a process for producing the polypeptides of the variants, as well as uses for the nucleic acid, polypeptide and antibodies to same in diagnosing human breast carcinoma, breast adenocarcinoma, cervix epidermoid carcinoma, cervix epitheloid carcinoma, colon adenocarcinoma, urinary bladder carcinoma, prostate carcinoma, esophagus epidermoid carcinoma and esophagus carcinoma.
Owner:VISGENEER
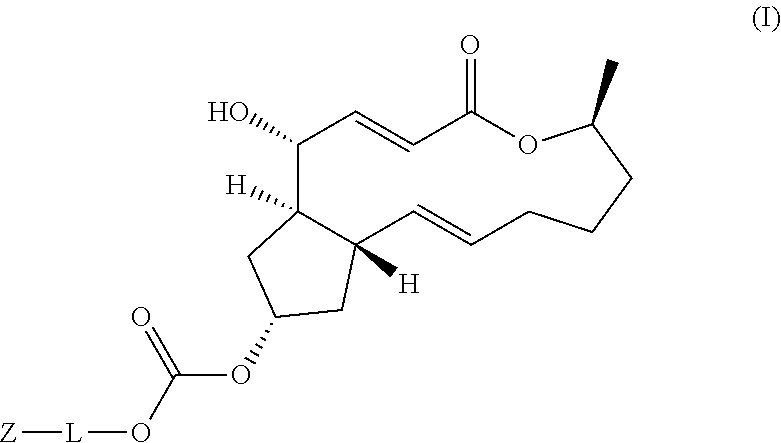
Brefeldin A Derivatives, Preparation Method and Use thereof
PendingUS20210403458A1Inhibit tumor growthMaintain stable propertiesOrganic active ingredientsOrganic chemistryDiseaseNasopharyngeal cancer
The present disclosure relates to the field of medicinal chemistry, and specifically to brefeldin A derivatives represent by Formula (I) and its use in the inhibition of tumor proliferation activity. The new compounds provided herein are prominent in the prevention or treatment of hyperproliferative diseases, including liver cancer, leukemia, breast cancer, colon adenocarcinoma, lung cancer, Bart's esophageal cancer, gastric cancer, cervical cancer, pancreatic cancer, kidney cancer, endometrial cancer, nasopharyngeal cancer, bone cancer, lymphoma, brain cancer, nerve cancer, oral cancer and colorectal cancer, and have the potential to be developed as novel antitumor agents.
Owner:OCEAN UNIV OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com