Process for producing trifluoro benzene acetic acid and sitagliptin
A technology of trifluorophenylacetic acid and sitagliptin, which is applied in the field of preparation of trifluorophenylacetic acid and sitagliptin, can solve the problems of unsuitability for industrial production, excessive three wastes, and expensive raw materials, and achieve low cost and less three wastes , the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
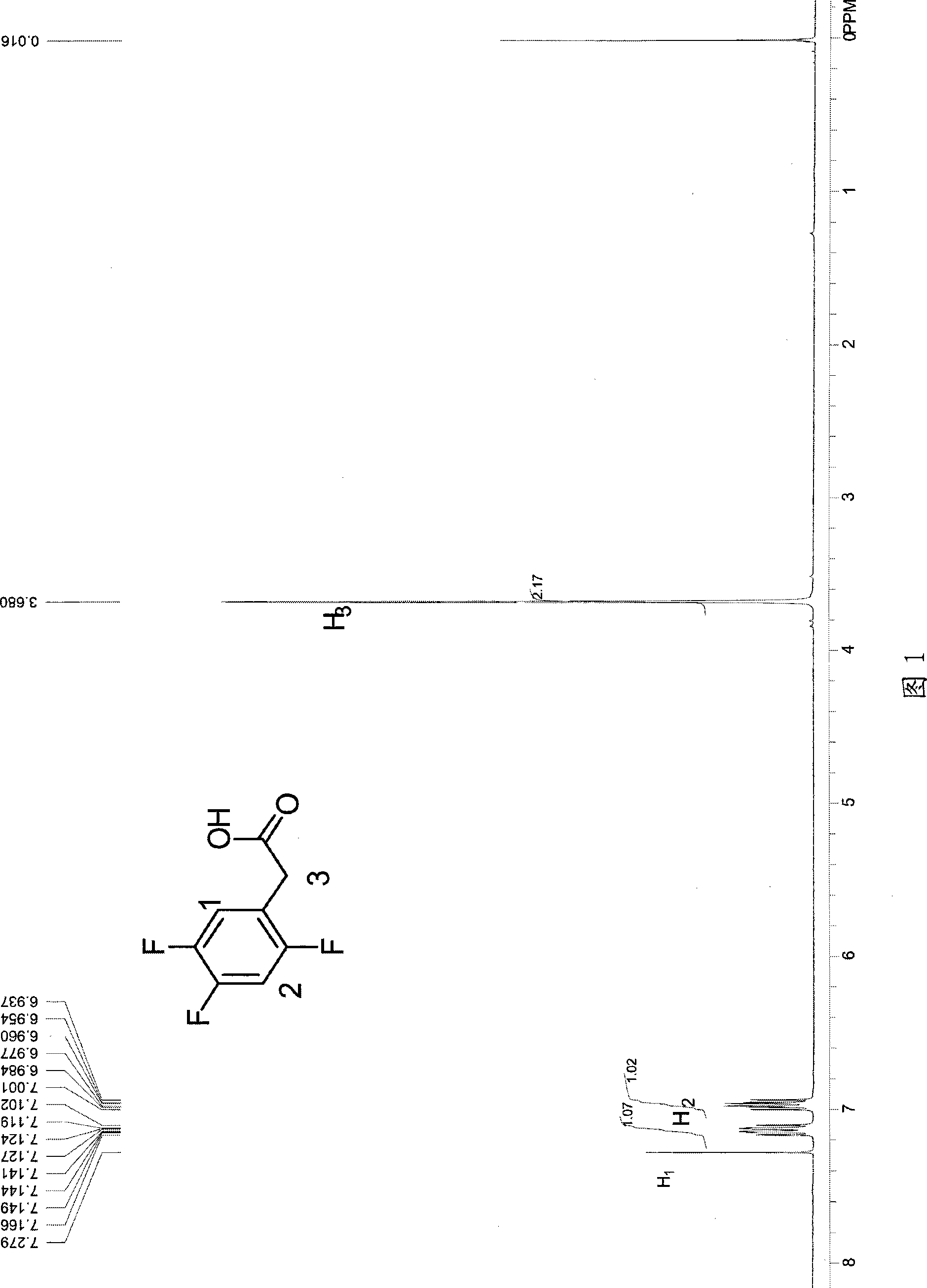
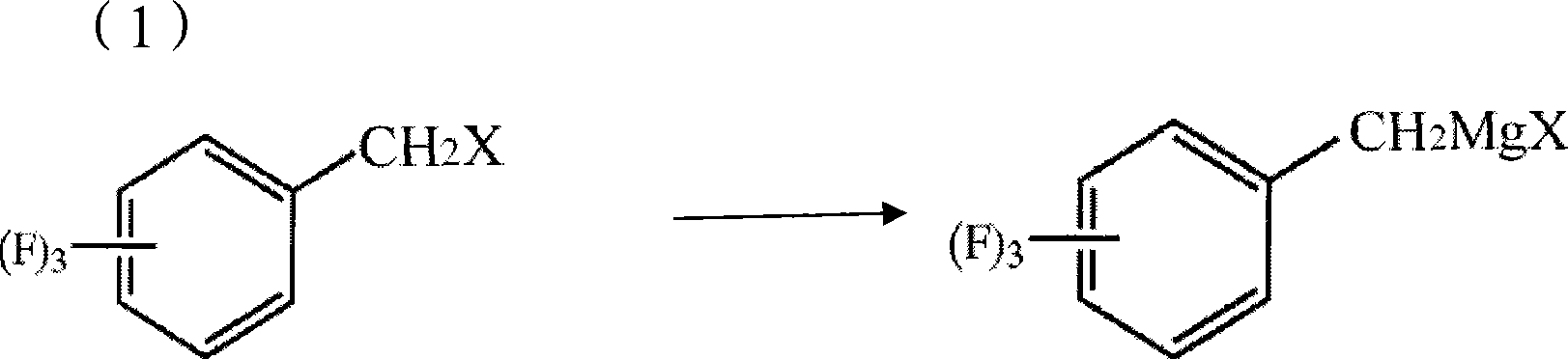
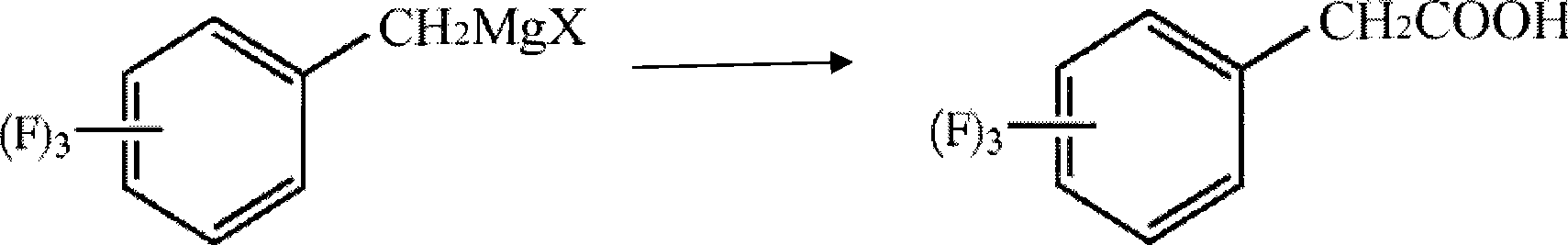
[0045]In a 250ml three-necked flask, 6.6g (0.275mol) of magnesium chips, 100g of ether, and 1ml of bromoethane (1%) were put into a 250ml three-necked flask under nitrogen protection. Raise the temperature to 40°C, start to add 7g (0.038mol) of 2,4,5-trifluorobenzyl chloride dropwise, and keep the reaction for 30 minutes after initiation, then continue to add 38g (0.212mol) of 2,4,5-trifluorobenzyl chloride dropwise After the dropwise addition, keep warm for 30 minutes, then cool to 5°C, start to feed carbon dioxide gas, control the flow of carbon dioxide to keep the internal temperature at 10-15°C, until the reaction system no longer releases heat and the temperature does not rise, stop the flow of carbon dioxide , and react at 10 °C for another 0.5 h. After the reaction, the reaction solution was added to 15% hydrochloric acid aqueous solution, hydrolyzed for half an hour, the aqueous layer was extracted twice with 100 g of methyl tert-butyl ether, the organic layers were co...
Embodiment 2
[0049] In a 250ml three-necked flask, 6g (0.25mol) of magnesium chips, 70g of ether and 70g of toluene were dropped under nitrogen protection, and 2g of the previous batch of Grignard reagent was used as an initiator (1.5%). Raise the temperature to 55°C, start to add 7g (0.038mol) of trifluorobenzyl chloride dropwise, and keep it warm for 30 minutes after initiation, then continue to add 38g (0.212mol) of 2,4,5-trifluorobenzyl chloride dropwise, and keep warm after the addition is complete 30 minutes, then cooled to 5°C, started to feed carbon dioxide gas, controlled the flow of carbon dioxide to maintain the internal temperature of 15-20°C, until the reaction system no longer exothermic, when the temperature no longer rises, stop the flow of carbon dioxide, and at 5°C React for another 1 hour. After the reaction, the reaction solution was added to 15% hydrochloric acid aqueous solution, hydrolyzed for half an hour, the aqueous layer was extracted twice with 100g toluene, the...
Embodiment 3
[0051] In a 500ml three-necked flask, 14.4g (0.6mol) of magnesium chips, 100g of ether and 150g of toluene were dropped under nitrogen protection, and 3g of the previous batch of Grignard reagent was used as an initiator (1.2%). Raise the temperature to 60°C, start to add 14g (0.076mol) of chlorobenzyl trifluorobenzyl dropwise, and keep the reaction for 30 minutes after initiation, then continue to add 76g (0.424mol) of 2,4,5-chlorobenzyl trifluorobenzyl dropwise, and keep warm after the addition is complete 60 minutes, then cooled to 5°C, began to feed carbon dioxide gas, controlled the flow of carbon dioxide to maintain the internal temperature of 5-10°C, until the reaction system no longer exothermic, when the temperature no longer rises, stop the flow of carbon dioxide, and at 5°C React for another 1 hour. After the reaction, the reaction solution was added to 15% hydrochloric acid aqueous solution, hydrolyzed for half an hour, the aqueous layer was extracted twice with 20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com