Process of producing pentafluorophenol
A technology of pentafluorophenol and pentafluorobromobenzene, which is applied in the field of pentafluorophenol production, can solve the problems that restrict the industrial production process of pentafluorophenol, the supply of raw materials cannot be guaranteed, and hexafluorobenzene is difficult to obtain. The effect of high purity and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
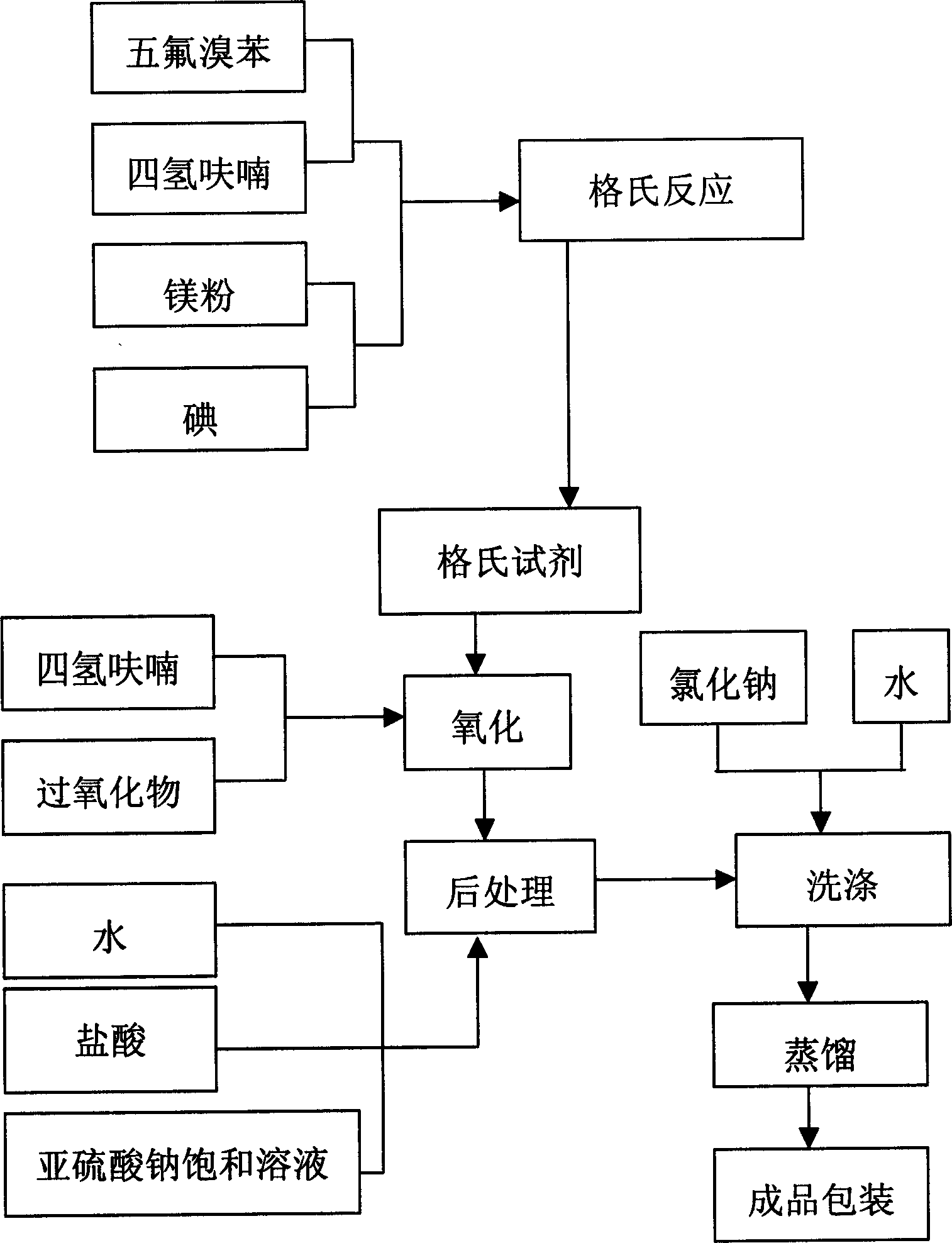
[0030] Refer to attached figure 1 . Install electric stirring paddle, dropping funnel, N 2 The inlet pipe, thermometer and five-ball reflux condenser are equipped with a calcium chloride drying tube on the condenser.
[0031] 12.6g (0.52mol) of magnesium chips inside the bottle, 1 small grain of iodine and 150ml of tetrahydrofuran, N 2 Heat up to 50°C under protection, add dropwise a solution made of 123.5g (0.50mol) pentafluorobromobenzene and 120ml tetrahydrofuran, the reaction temperature is 50-55°C, keep warm for 2 hours after addition, and cool to room temperature. The above reaction is a Grignard reaction, the product is a Grignard reagent, and the reaction equation is:
[0032]
[0033] Stir in a 1000ml three-necked bottle, put a thermometer, and a dropping funnel, put 200ml of tetrahydrofuran and 63.6g (0.6mol) of 85% tert-butyl hydroperoxide into the bottle, cool to -15°C, and add dropwise at -15 to 10°C A good Grignard reagent was added and incubated for 1 hou...
Embodiment 1-2
[0038] Pre-weigh 130kg of magnesium chips and 500g of iodine, measure 150L of tetrahydrofuran, add them into a 500L reaction pot, open the valve of the stirring and condenser, connect the drying pipe, fill the air in the kettle with nitrogen, and maintain an appropriate nitrogen flow rate, Heat up to 50°C and stir for 30 minutes, add 4-6% of the total amount of pentafluorobromobenzene tetrahydrofuran solution prepared in advance to 1300kg: 120L from the high-level tank, wait for the reaction in the pot to start, and the internal temperature will rise to 50-55°C , the remaining pentafluorobromobenzenetetrahydrofuran solution was added, and kept at this temperature for 2 hours. After the end, the jacket was opened to cool water and cooled to room temperature for use.
[0039] Put 200L tetrahydrofuran and 640kg peroxide into a 1000L dry reaction pot, and when it is cooled to -15°C, add the Grignard reagent to be used in the previous step, and control the dropping temperature at -1...
Embodiment 1-3~ Embodiment 1-15
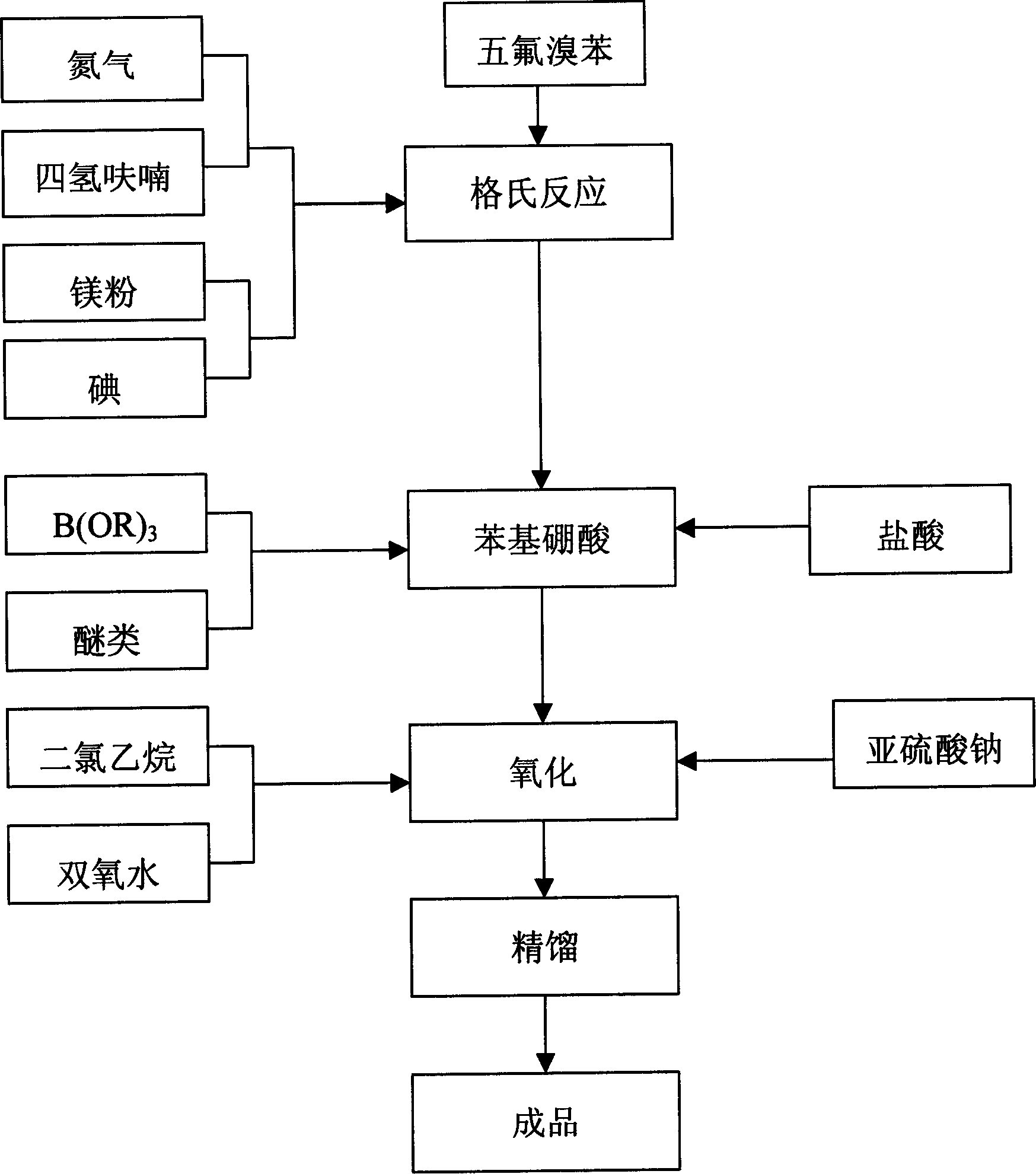
[0042] Among the above examples, examples 1-3 to 1-12 use tetrahydrofuran as the organic solvent. Embodiment 1-13 uses ether as the organic solvent, embodiment 1-14 uses propyl ether as the organic solvent, embodiment 1-15 uses isopropyl ether as the organic solvent, and embodiment 1-16 uses butyl ether as the organic solvent.
[0043] Embodiment 1-3~Example 1-8 Grignard reaction temperature 50~55 ℃, embodiment 1-9~Example 1-11 Grignard reaction temperature 57~63 ℃, embodiment 1-2~embodiment 1- 14 The Grignard reaction temperature is 39-45°C, the Grignard reaction temperature is 32-38°C in Example 1-15, and the Grignard reaction temperature is 62-68°C in Example 1-16.
[0044]The mol ratio of embodiment 1-3~embodiment 1-8 pentafluorobromobenzene and magnesium is 1: 1.2, the mol ratio of embodiment 1-9~embodiment 1-11 pentafluorobromobenzene and magnesium is 1: 0.95, The mol ratio of embodiment 1-12~embodiment 1-14 pentafluorobromobenzene and magnesium is 1: 1.0, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com