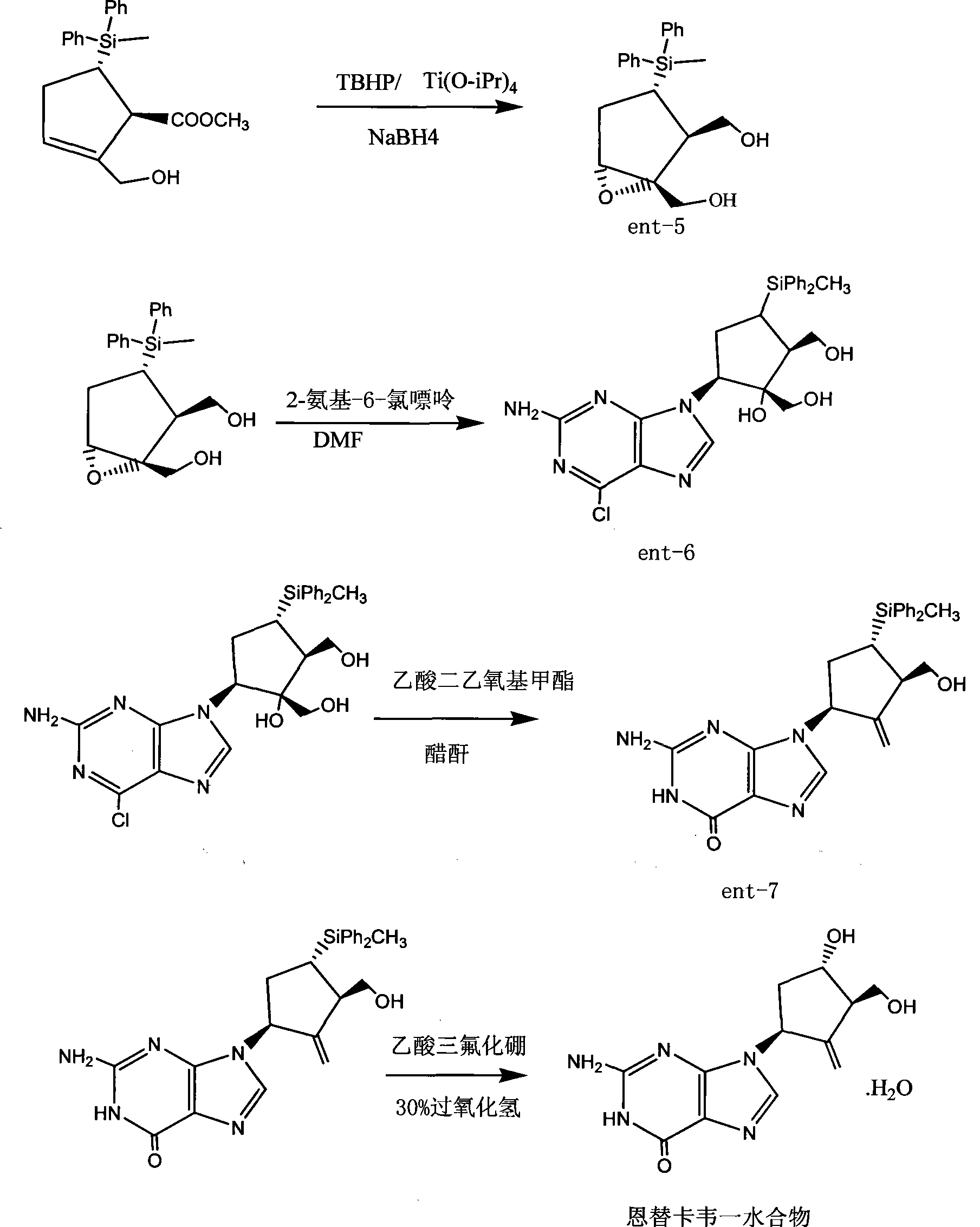
Industrialization production process of entecavir-monohydrate
A technology of entecavir and production process, applied in the field of organic synthetic drugs, can solve problems such as being unsuitable for industrial production, the method is not simple enough, and achieve the effects of reduced synthesis cost, low cost and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
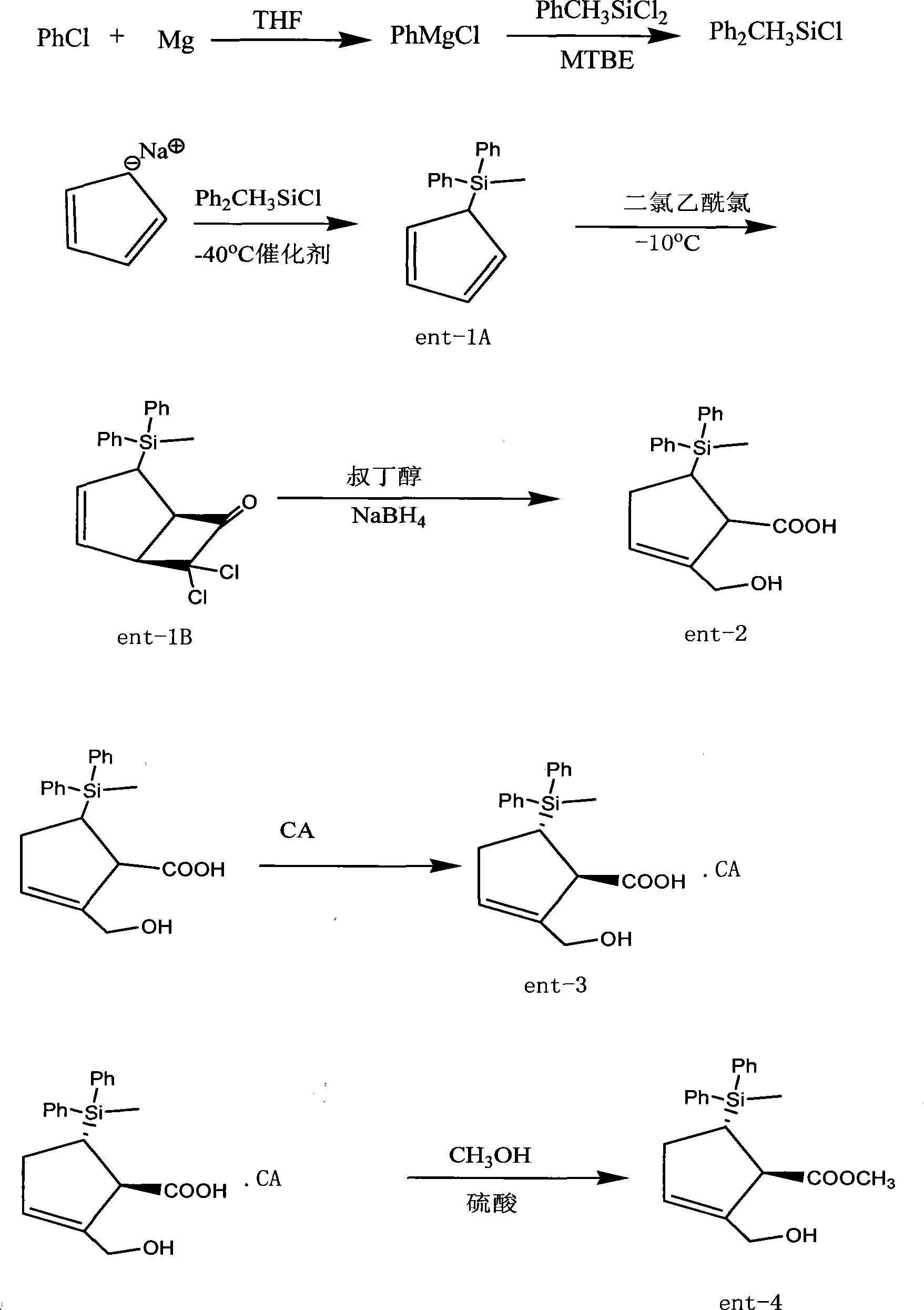
[0044] Embodiment 1, the preparation of methyl diphenylchlorosilane:
[0045] In a 500ml four-necked bottle, add 50ml of THF dried with sodium in advance, 16.7g (0.687mol) of magnesium chips, 5ml of chlorobenzene, one grain of iodine is added to trigger, the temperature rises to 70°C, and 200ml of THF solution of 65ml of chlorobenzene is added dropwise , keep slightly boiling, after addition, reflux reaction for 6 hours, after completion of reaction, seal and store for later use.
[0046] Add 400ml of THF, 3g of cuprous cyanide, 133g (0.695mol) of methylphenyldichlorosilane into another 1000ml four-necked bottle, raise the temperature to 50°C, add the above-mentioned Grignard solution dropwise at this temperature, and complete the addition. Insulate and react for 3 hours, add 200ml of n-hexane, stir for 30 minutes, filter, concentrate the filtrate under reduced pressure, collect about 112g of 128-133°C / 670pa fraction, and the yield is about 70%.
Embodiment 2
[0047] The preparation of embodiment 2, ent-1A:
[0048] In a 500ml four-necked bottle, add 100ml of THF dried with sodium in advance, 20.5g (0.088mol) of methyldiphenylchlorosilane, blow nitrogen, cool to -40°C, and dissolve 44ml (0.088mol) of cyclopentadiene sodium solution (2M inTHF) was fully dissolved with 60ml THF, and 1ml of catalyst was added, and stirred evenly.
[0049] Under the protection of nitrogen, add cyclopentadiene sodium solution dropwise for about two hours, keep warm for 2 hours, stop refrigeration, let the reaction solution gradually warm up to 0°C, carefully add 80ml of water, stir for 30 minutes, separate layers, and use 50ml× Wash twice with 2 water, dry over anhydrous sodium sulfate for 2 hours, filter, and concentrate to dryness under reduced pressure at 50°C to obtain about 22.5 g of dark red oil ent-1A
Embodiment 3
[0050] Embodiment 3, the preparation of ent-1B:
[0051]In a 500ml dry four-neck flask, add 22.5g (0.0857mol) of ent-1A, 200ml of n-hexane, lower the temperature to -10°C under the protection of nitrogen, add 25.3g (0.171mol) of dichloroacetyl chloride dropwise, after the addition is complete, stir In 30 minutes, 17.3 g (0.171 mol) of triethylamine was added dropwise, and the addition was completed in 90 minutes. The reaction solution was slowly raised to room temperature, and stirred at room temperature for 12 hours. Wash once with 50ml of sodium solution, wash twice with 50ml×2 water, dry over anhydrous sodium sulfate for 2 hours, filter, and concentrate to dryness at 50°C to obtain about 32g of dark red oil ent-1B
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com