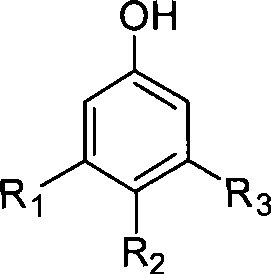
Method for preparing fluorophenol
A phenol and fluorine-substituted technology, which is applied in the field of preparation of trifluorophenol, can solve the problems of inconvenient process flow and post-processing, not conforming to the establishment of a conservation-oriented society, and unsuitable for industrial production, and achieves low cost, stable quality, and post-processing easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
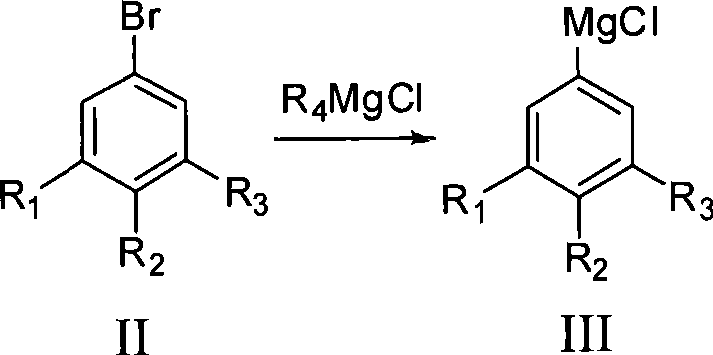
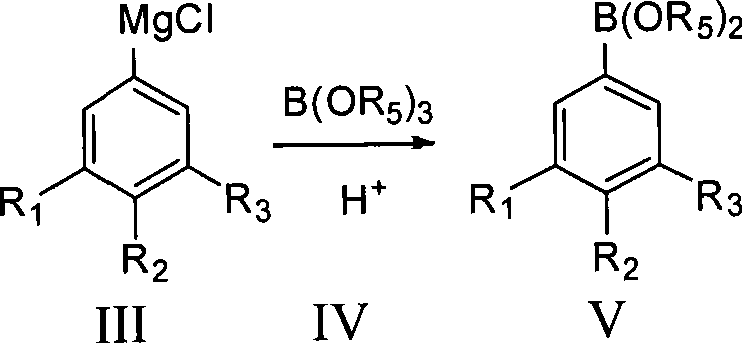
[0033] The preparation method of fluorophenol of the present invention, take fluorobromophenyl bromobenzene as raw material, and R 4 MgCl undergoes Grignard exchange to generate fluorophenylmagnesium chloride, and then reacts with B(OR 5 ) 3 The reaction produces fluorophenylboronic acid ester, which is hydrolyzed under acidic conditions to obtain fluorophenylboronic acid. Fluorinated phenylboronic acid is oxidized with hydrogen peroxide to obtain crude fluorophenol, and the product fluorophenol is obtained after post-treatment, and its content is >99.9%. The present invention prepares the Grignard reagent of fluorobromobenzene by Grignard exchange reaction, improves the conversion rate, reduces the impurity content in the Grignard reagent, obtains the Grignard reagent of high-purity fluorobromobenzene, thereby greatly improves The purity and yield of the final product.
[0034] Beneficial effects of the present invention: the preparation method of fluorophenol of the prese...
Embodiment 1
[0037] Example 1 Preparation of i-PrMgCl
[0038] 69g (2.89mol) of Mg and 200ml THF were added to a 2000ml four-necked flask, and a mixed solution of 216g i-PrCl (2.75mol) and 770ml THF was added dropwise at 30°C under stirring. After the dropwise addition, stir for 0.5 to 1 hour and keep warm for later use.
Embodiment 2
[0039] Example 2 Preparation of 3,4,5-trifluorophenylboronic acid
[0040] Add 527.5g of 3,4,5-trifluorobromobenzene (2.5mol) and 1100ml of THF into a 5000ml four-neck flask, stir for 15min, cool down to -20°C and add i-PrMgCl dropwise, after the dropwise addition, keep stirring for 2h, dropwise add 286g of trimethyl borate (2.75mol), keep warm for 2 hours after the dropwise addition, add 365g of 30% HCl dropwise, keep warm for 2 hours after the dropwise addition, evaporate THF, add 600ml of EtOAc and 500ml of water to dissolve and separate the liquid, and extract the water phase with 200ml of EtOAc The organic phases were combined, EtOAc was distilled off, and 350 ml of n-hexane was beaten to obtain 350 g of 3,4,5-trifluorophenylboronic acid with a content of 99.8% and a yield of 79.5%. The boiling point is 132°C / 20mmHg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com