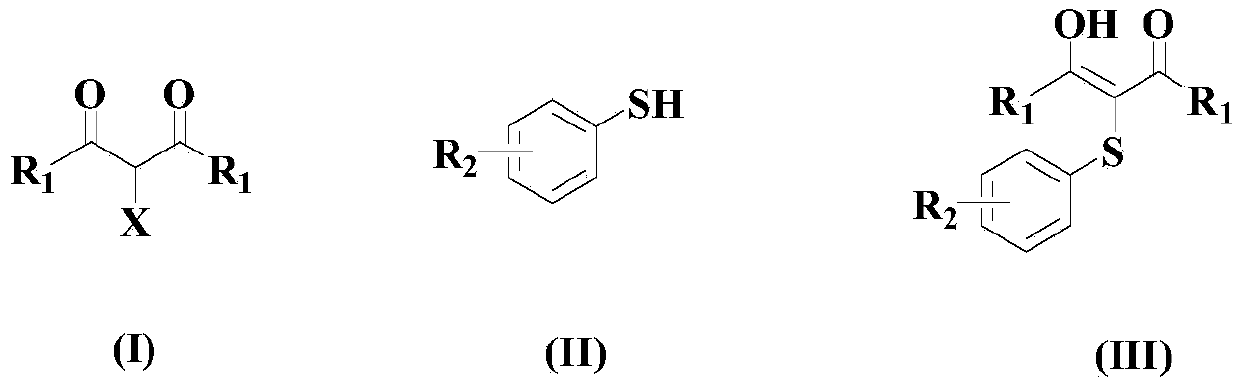
Synthetic method of medical intermediate carbonyl-replacement aryl thioether compound
A synthesis method and technology of aryl sulfide, which is applied in the synthesis of sulfide compounds and the synthesis of carbonyl-substituted aryl sulfide compounds, can solve the problems of low reaction yield and reaction process to be optimized, and achieve broad market prospects, The effect of mild reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
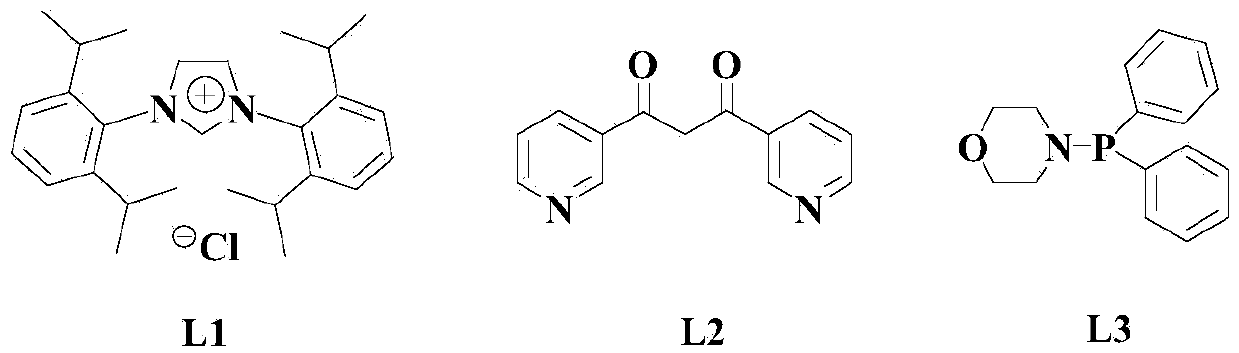
[0044] At room temperature and under a nitrogen atmosphere, to an appropriate amount of organic solvent (a mixture of chlorobenzene and DMSO with a volume ratio of 1:2), add 100mmol of the compound of the above formula (I), 140mmol of the compound of the above formula (II), and 10mmol of the catalyst (8mmolCu( PPh 3 ) 2 NO 3 With 2mmolNi(acac) 2 The mixture), 5mmol of organic ligand L1, 10mmol of activator p-methoxyphenyl tellurium oxide and 200mmol of basic cesium carbonate, then heated to 60°C, and stirred at this temperature for 8 hours;
[0045] After the reaction is completed, the reaction system is naturally cooled to room temperature, filtered, and 5% by mass hydrochloric acid aqueous solution is added to the filtrate, shaken thoroughly, and then ethyl acetate is added for extraction 2-3 times, and the organic phases are combined and decompressed. After distillation, the residue was subjected to 300-400 mesh silica gel column chromatography, and washed with acetone...
Embodiment 2
[0048]
[0049] Under room temperature and nitrogen atmosphere, to an appropriate amount of organic solvent (a mixture of chlorobenzene and DMSO with a volume ratio of 1:2), add 100mmol of the compound of the above formula (I), 170mmol of the compound of the above formula (II), and 12mmol of catalyst (8mmolCu( PPh 3 ) 2 NO 3 With 4mmolNi(acac) 2 ), 8mmol of organic ligand L1, 15mmol of activator p-methoxyphenyl tellurium oxide and 250mmol of basic cesium carbonate, then the temperature is raised to 75°C, and the reaction is stirred at this temperature for 6 hours;
[0050] After the reaction is completed, the reaction system is naturally cooled to room temperature, filtered, the filtrate is added to the filtrate with a 7% mass percent hydrochloric acid aqueous solution, fully shaken, and then ethyl acetate is added for extraction 2-3 times, and the organic phases are combined and decompressed After distillation, the residue was subjected to silica gel column chromatography of 300-...
Embodiment 3
[0053]
[0054] At room temperature and under a nitrogen atmosphere, to an appropriate amount of organic solvent (a mixture of chlorobenzene and DMSO with a volume ratio of 1:2), 100mmol of the compound of the above formula (I), 200mmol of the compound of the above formula (II), and 16mmol of the catalyst (12mmolCu( PPh 3 ) 2 NO 3 With 4mmolNi(acac) 2 ), 10mmol of organic ligand L1, 20mmol of activator p-methoxyphenyl tellurium oxide and 300mmol of basic cesium carbonate, then the temperature is raised to 90°C, and the reaction is stirred at this temperature for 5 hours;
[0055] After the reaction is completed, the reaction system is naturally cooled to room temperature, filtered, and a 10% aqueous hydrochloric acid solution is added to the filtrate, shaken thoroughly, and then ethyl acetate is added for extraction 2-3 times, and the organic phases are combined and decompressed After distillation, the residue was subjected to silica gel column chromatography of 300-400 mesh, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com