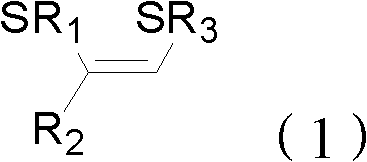
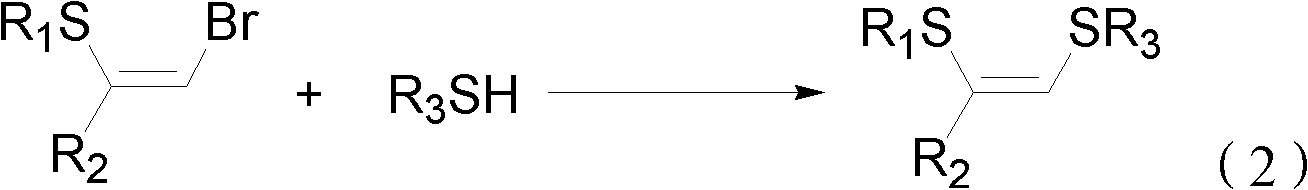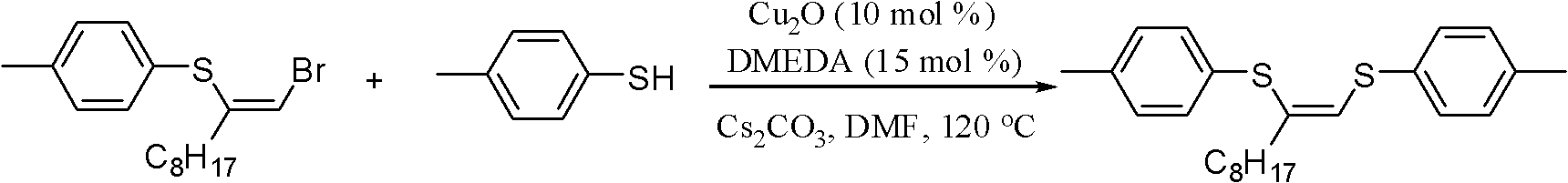Method for preparing (Z)-1,2-disulfide-1-olefin by catalysis of metal copper salt
A metal copper salt, a technology for catalytic preparation, which is applied in chemical instruments and methods, formation/introduction of mercapto/thioether groups, preparation of thioethers, etc., to achieve the effects of cheap and easy-to-obtain reaction raw materials, simple preparation methods, and diverse product structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Under the protection of nitrogen, add (Z)-1-bromo-2-p-methylphenylsulfide-1-decene 171mg, Cs to the reaction tube 2 CO 3 326mg, Cu 2 O 7mg, DMEDA 8μL, p-methylthiophene 124mg, DMF 2mL, mix well, react at 120°C for 24h. After the reaction, it was cooled to room temperature, and then 15 mL of water was added to the reactant, extracted with ethyl acetate, dried with anhydrous sodium sulfate, concentrated, and passed through a silica gel column (the volume ratio of petroleum ether to ethyl acetate was 50:1) to obtain The product (Z)-1,2-dimethylphenylsulfide-1-decene, the resulting product is 157 mg, and the yield is 82%. 1 H NMR(400MHz, CDCl 3 ): δ7.32-7.27(m, 4H), 7.13-7.08(m, 4H), 6.45(s, 1H), 2.32(s, 3H), 2.31(s, 3H), 2.20(t, J=7.2 Hz, 2H), 1.49-1.46 (m, 2H), 1.29-1.20 (m, 10H), 0.87 (t, J=6.8 Hz, 3H); 13 C NMR(100MHz, CDCl 3 ): δ137.12, 137.11, 134.7, 132.8, 131.5, 130.4, 130.3, 130.1, 130.0, 128.8, 37.2, 32.1, 29.6, 29.5, 29.1, 28.8, 22.9, 21.4, 21.3, 14.4.
Embodiment 2
[0027]
[0028] Under the protection of nitrogen, add (Z)-1-bromo-2-p-methylphenylsulfide-1-decene 171mg, Cs to the reaction tube 2 CO 3 326mg, Cu 2 O 7mg, TMEDA 15μL, p-cresol 124mg, DMF 2mL, react at 120°C for 24h. Cool to room temperature, add 15 mL of water, extract with ethyl acetate, dry with anhydrous sodium sulfate, concentrate, and pass through a silica gel column (the volume ratio of petroleum ether to ethyl acetate is 50:1) to obtain product (Z)-1, 2 -Di-p-methylphenyl sulfide-1-decene, the resulting product is 148 mg, and the yield is 77%.
Embodiment 3
[0030]
[0031] Under the protection of nitrogen, add (Z)-1-bromo-2-p-methylphenylsulfide-1-decene 171mg, Cs to the reaction tube 2 CO 3 326mg, Cu 2 O 7mg, DMEDA 8μL, p-toluol 124mg, THF 2mL, react at 120°C for 24h. Cool to room temperature, add 15 mL of water, extract with ethyl acetate, dry with anhydrous sodium sulfate, concentrate, and pass through a silica gel column (the volume ratio of petroleum ether to ethyl acetate is 50:1) to obtain product (Z)-1, 2 -Di-p-methylphenyl sulfide-1-decene, the resulting product is 144 mg, and the yield is 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com