Method for preparing diaryl disulfide and diaryl diselenide under catalysis of aqueous phase
A technology for catalysts to catalyze sulfur and catalysts, applied in chemical instruments and methods, formation/introduction of mercapto/sulfide groups, organic chemical methods, etc., can solve problems such as cumbersome preparation process, high cost, and reduced reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
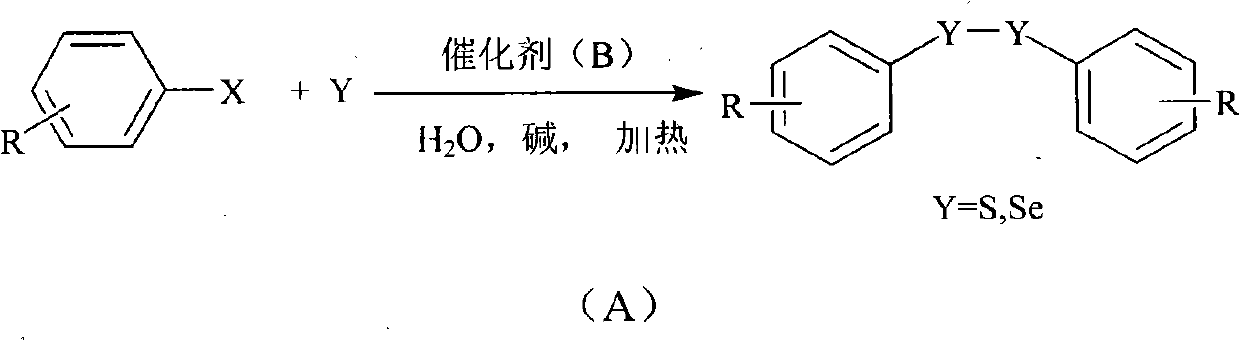
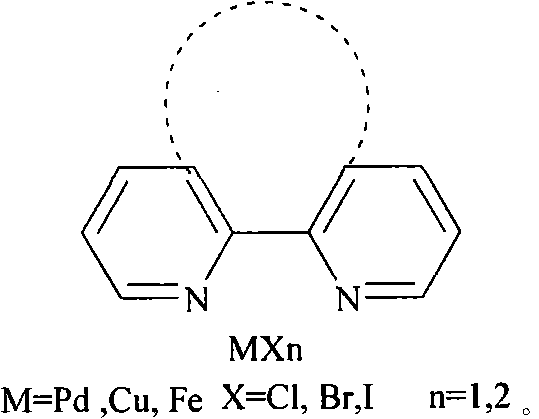
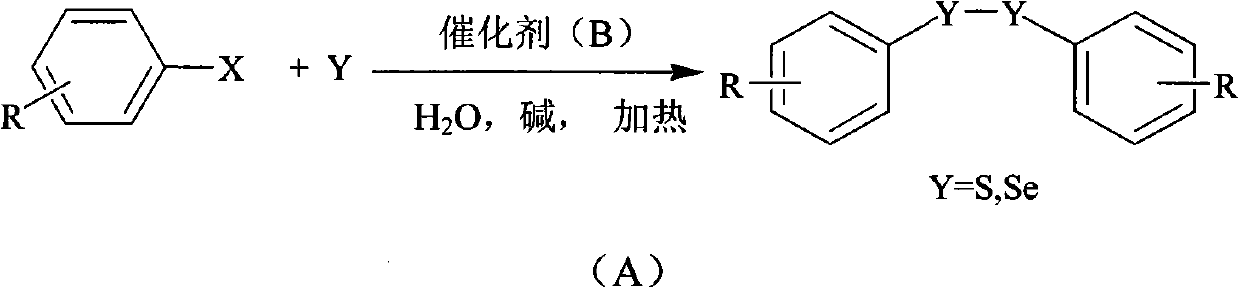
[0026] Example 1: Preparation of diphenyl disulfide from iodobenzene: Add 1 mmol of iodobenzene, 0.05 mmol of copper complex, 1 mmol of sodium hydroxide, 3.0 mmol of sulfur powder, 0.1 mmol of tetrabutylammonium fluoride, and 2 mL of water into a reaction vessel . React in an oil bath at 100°C for 30 hours and cool to room temperature. The product was extracted with ethyl acetate, concentrated under reduced pressure, and purified by column chromatography to obtain a light yellow oily product with a yield of 95%.
Embodiment 2
[0027] Example 2: Preparation of p-methylphenylbenzene disulfide by p-methyliodobenzene: add 0.5mmol of iodobenzene, 0.5mmol of p-methyliodobenzene, 0.05mmol of copper complex, 1mmol of sodium hydroxide, sulfur powder 3.0mmol, tetrabutylammonium fluoride 0.1mmol, water 2mL. React in an oil bath at 100°C for 30 hours and cool to room temperature. The product was extracted with ethyl acetate and concentrated under reduced pressure. The product was purified by column chromatography to obtain a light yellow liquid with a yield of 93%.
Embodiment 3
[0028] Embodiment 3: p-methoxyphenylbenzene disulfide is prepared from p-methoxy iodobenzene: the preparation method is the same as in Example 2, adding 0.5 mmol of iodobenzene and 0.5 mmol of p-methoxy iodobenzene to obtain a colorless liquid, producing The rate is 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com