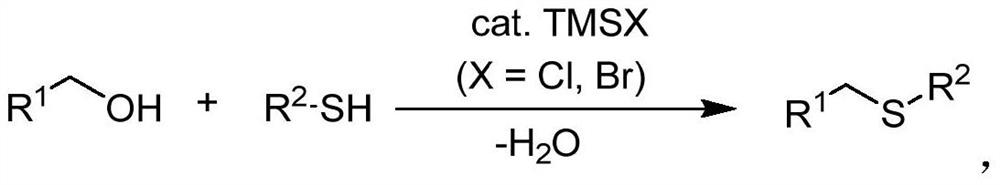Synthetic method of asymmetric thioether
A synthetic method and asymmetric technology, applied in the formation/introduction of mercapto/thioether groups, thioether preparation, organic chemistry, etc., can solve the problems of limited application range, large amount of waste, poor stability, etc., and shorten the synthesis steps , easy to operate, good selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
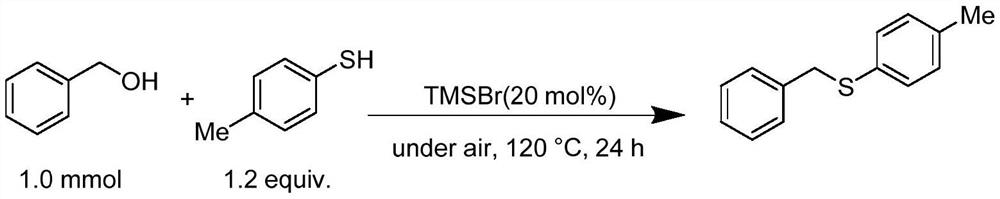
[0020] Preparation of p-methylphenyl benzyl sulfide with benzyl alcohol and p-cresyl thiophenol
[0021]
[0022] Benzyl alcohol (108.1mg, 1.0mmol), p-cresylthiophenol (149.0mg, 1.2equiv.) and bromotrimethylsilane (30.6mg, 20mol%) were added successively to the tubular reactor, and it was directly sealed under air without Under solvent conditions, heat at 120°C for 24h. After the completion of the reaction was monitored by TLC, the product was separated and purified by column chromatography. The isolated yield was 86%. 1 H NMR (400MHz, CDCl 3 ):δ7.27-7.18(m,4H),7.18-7.08(m,3H),7.00(s,2H),4.00(s,2H),2.24(s,3H). 13 C NMR (101MHz, CDCl 3 ): δ137.82, 136.56, 132.51, 130.72, 129.63, 128.85, 128.44, 127.08, 39.81, 21.08.
Embodiment 2
[0024] Preparation of p-methylphenyl benzyl sulfide with benzyl alcohol and p-cresyl thiophenol
[0025]
[0026] Benzyl alcohol (108.1mg, 1.0mmol), p-cresylthiophenol (149.0mg, 1.2equiv.) and trimethylchlorosilane (21.7mg, 20mol%) were successively added into the tubular reactor, and it was directly sealed under air without Under solvent conditions, heat at 120°C for 24h. After the completion of the reaction was monitored by TLC, the product was separated and purified by column chromatography. The isolated yield was 41%. 1 H NMR (400MHz, CDCl 3 ):δ7.27-7.18(m,4H),7.18-7.08(m,3H),7.00(s,2H),4.00(s,2H),2.24(s,3H). 13 C NMR (101MHz, CDCl 3 ): δ137.82, 136.56, 132.51, 130.72, 129.63, 128.85, 128.44, 127.08, 39.81, 21.08.
Embodiment 3
[0028] Preparation of p-methylphenyl benzyl sulfide with benzyl alcohol and p-cresyl thiophenol
[0029]
[0030] Benzyl alcohol (108.1mg, 1.0mmol), p-cresylthiophenol (149.0mg, 1.2equiv.) and bromotrimethylsilane (30.6mg, 20mol%) were added successively in the tubular reactor, and the 2 Under the condition of sealed and solvent-free heating at 120 ° C for 24 hours. After the completion of the reaction was monitored by TLC, the product was separated and purified by column chromatography. The isolated yield was 76%. 1 H NMR (400MHz, CDCl 3 ):δ7.27-7.18(m,4H),7.18-7.08(m,3H),7.00(s,2H),4.00(s,2H),2.24(s,3H). 13 CNMR (101MHz, CDCl 3 ): δ137.82, 136.56, 132.51, 130.72, 129.63, 128.85, 128.44, 127.08, 39.81, 21.08.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com