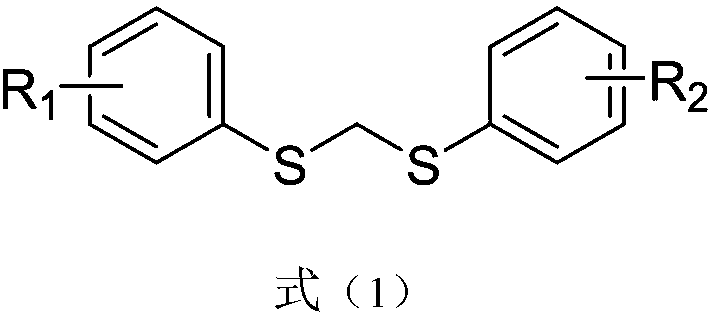
Thioether compound and synthetic method and application thereof
A technology of disulfide compounds and compounds, applied in the formation/introduction of mercapto/thioether groups, thioether preparation, organic chemistry, etc., can solve the problems of easy pollution of the environment, harsh conditions, and not very mild reaction conditions, and achieve easy The effect of industrialized production, simple reaction equipment and simple conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
[0036] The synthesis of embodiment 1 diphenylthiomethane
[0037] Take a 15mL pressure-resistant reaction tube, add 87mg (0.4mmol) of diphenyl disulfide, 260mg (0.8mmol) of cesium carbonate, 18-crown-6210mg (0.8mmol), 6mL of anhydrous acetone, and stir at 40°C for 24h . The mixture was stirred at 40°C for 24 hours under an air atmosphere. The reaction was quenched with saturated brine, extracted with ethyl acetate (EtOAc), washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The residue was then purified by silica gel flash column chromatography with petroleum ether to obtain the desired product diphenylthiomethane 86 mg, yield 92%, as a white solid.
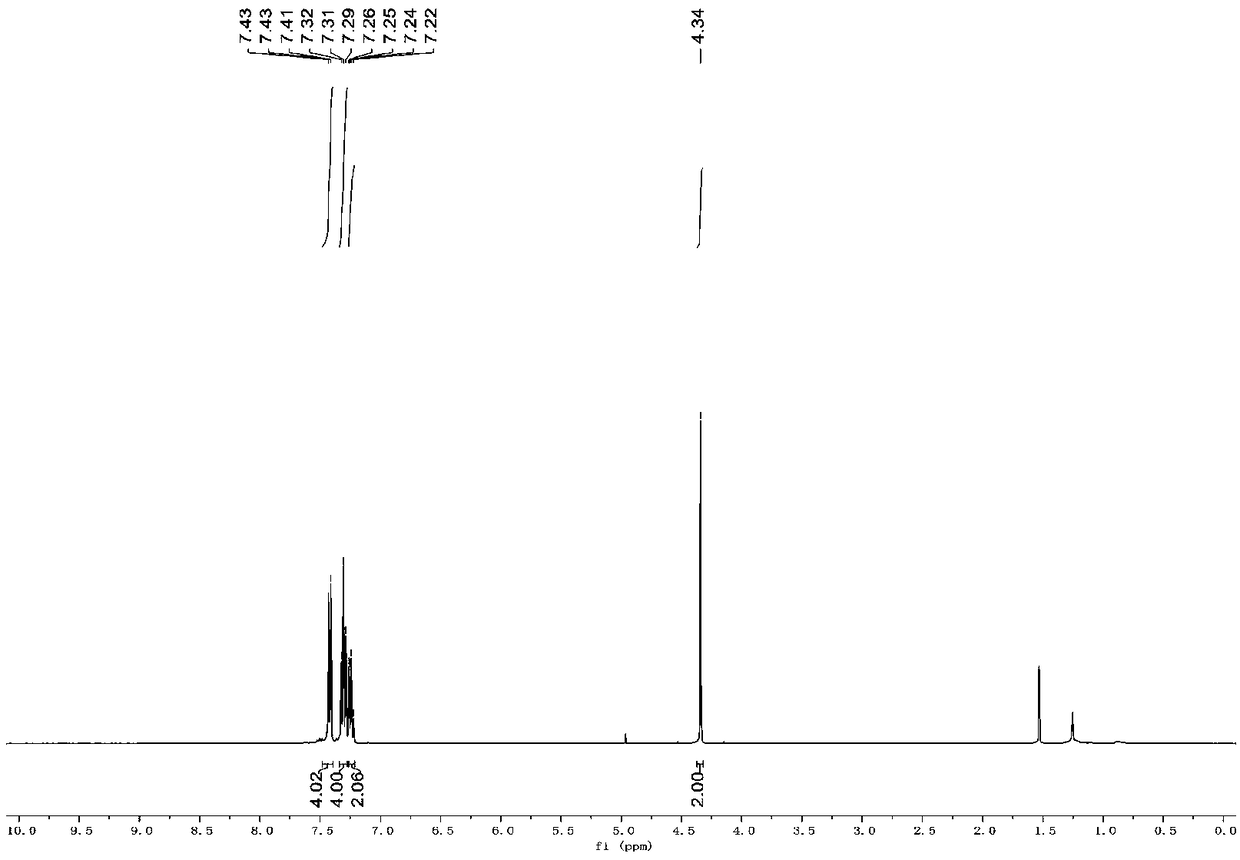
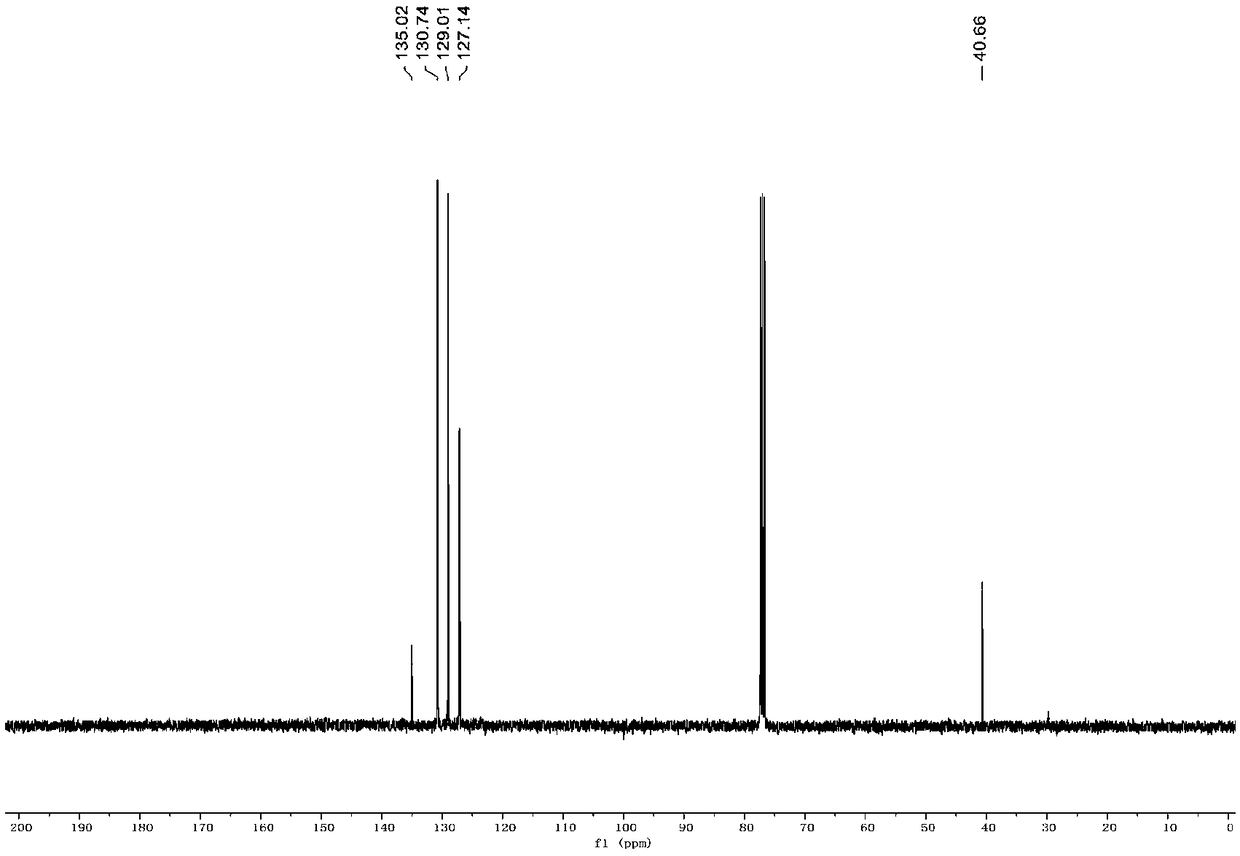
[0038] figure 1 It is the NMR H spectrum of diphenylthiomethane prepared in the present embodiment. figure 2 It is the NMR C spectrum of diphenylthiomethane prepared in the present embodiment. from figure 1 and 2 It can be seen from 1 H NMR (400MHz, CDCl3) δ7.43-7.41 (m, 4H), 7.31-7.29 (m, 4H), ...
Embodiment 2
[0043] Take a 15mL pressure-resistant reaction tube, add 87mg (0.4mmol) of diphenyl disulfide, 170mg (0.8mmol) of potassium phosphate, 18-crown-6210mg (0.8mmol), 6mL of anhydrous acetone, and stir at 40°C for 24h . The mixture was stirred at 40°C for 24 hours under an air atmosphere. The reaction was quenched with saturated brine, extracted with EtOAc, washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The residue was then purified by silica gel flash column chromatography with petroleum ether to obtain the desired product diphenylthiomethane 51 mg, yield 54%, as a white solid.
Embodiment 3
[0045] Take a 15mL pressure-resistant reaction tube, add 87mg (0.4mmol) of diphenyl disulfide, 111mg (0.8mmol) of potassium carbonate, 18-crown-6210mg (0.8mmol), 6mL of anhydrous acetone, and stir at 100°C for 24h . The mixture was stirred at 100° C. for 8 hours under an air atmosphere. The reaction was quenched with saturated brine, extracted with EtOAc, washed with anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure. The residue was then purified by silica gel flash column chromatography with petroleum ether to obtain the desired product diphenylthiomethane 65.5 mg, yield 70%, as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com