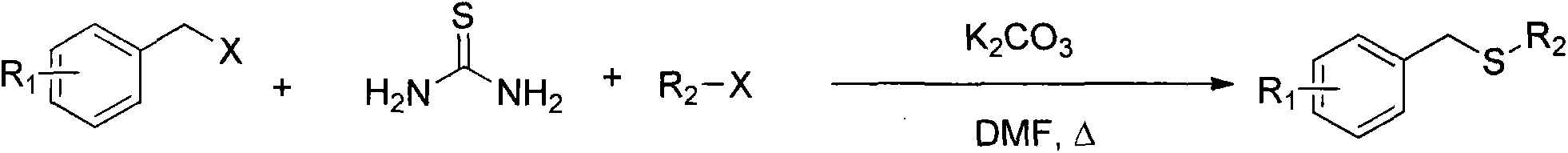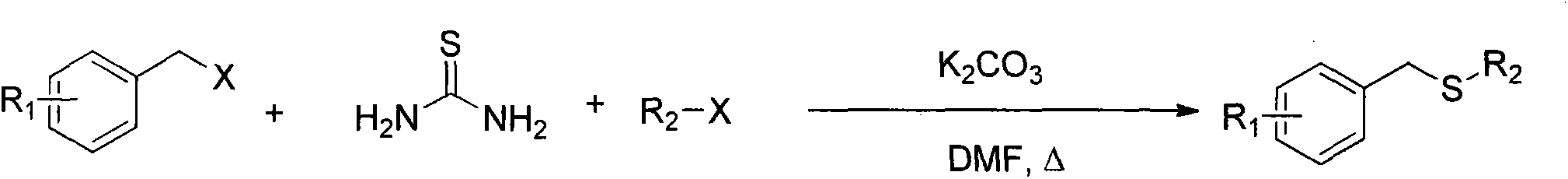Method for synthesizing benzyl alkyl sulfur ether
A technology for synthesizing benzyl alkyl sulfide and alkyl halide, applied in the field of synthesis of benzyl alkyl sulfide, can solve the problems of unfriendly environment, toxicity, long reaction time and the like, shorten the reaction time and improve the yield. efficiency and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of benzyl dodecyl sulfide
[0023] Benzyl bromide 169mg (1mmol), 1-bromododecane 273mg (1.1mmol), thiourea 91mg (1.2mmol) and potassium carbonate 414mg (3mmol) are added in the reaction bottle that 5mLDMF is housed, and reaction temperature is set to 100°C, heating and stirring. The progress of the reaction was monitored by gas chromatography, and the reaction was stopped when the benzyl bromide was consumed. Naturally cooled to room temperature, concentrated and evaporated to remove the solvent, dissolved in dichloromethane, washed three times with water, combined organic phases, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain a crude product. Purify by silica gel column, eluent: n-hexane, and isolate benzyl dodecyl sulfide.
[0024] Benzyl dodecyl sulfide is a colorless oil with a yield of 87.6%.
[0025] GC-MS: C 19 h 32 S[M+H] + The calculated value of is 292.2225 and the measured value is 291.7474.
[0026] 1 H NMR (4...
Embodiment 2
[0029] Preparation of Benzyl Octyl Sulfide
[0030] 169 mg (1 mmol) of benzyl bromide, 249 mg (1.3 mmol) of 1-bromooctane, 91 mg (1.2 mmol) of thiourea and 414 mg (3 mmol) of potassium carbonate were added to a reaction flask containing 5 mL of DMF, and the reaction temperature was set to 100 ℃, heating and stirring. The progress of the reaction was monitored by gas chromatography, and the reaction was stopped when the benzyl bromide was consumed. Naturally cooled to room temperature, concentrated and evaporated to remove the solvent, dissolved in dichloromethane, washed three times with water, combined organic phases, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain a crude product. Purify by silica gel column, eluent: n-hexane, and isolate benzyl octyl sulfide.
[0031] Benzyl octyl sulfide is a colorless oil with a yield of 87.1%.
[0032] GC-MS: C 15 h 24 S[M+H] + The calculated value of is 236.1599 and the measured value is 235.9009.
[0...
Embodiment 3
[0036] Preparation of Benzyl Hexyl Sulfide
[0037] Add 169 mg (1 mmol) of benzyl bromide, 327 mg (2 mmol) of 1-bromohexane, 91 mg (1.2 mmol) of thiourea and 414 mg (3 mmol) of potassium carbonate to a reaction flask containing 5 mL of DMF, and set the reaction temperature to 100°C , heating and stirring. The progress of the reaction was monitored by gas chromatography, and the reaction was stopped when the benzyl bromide was consumed. Naturally cooled to room temperature, concentrated and evaporated to remove the solvent, dissolved in dichloromethane, washed three times with water, combined organic phases, dried over anhydrous magnesium sulfate, filtered and concentrated to obtain a crude product. Purify by silica gel column, eluent: n-hexane, and isolate benzyl hexyl sulfide.
[0038] Benzylhexyl sulfide is a colorless oily substance with a yield of 92.3%.
[0039] GC-MS: C 12 h 20 S[M+H] + The calculated value is 208.1286, and the measured value is 207.8443.
[0040]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com