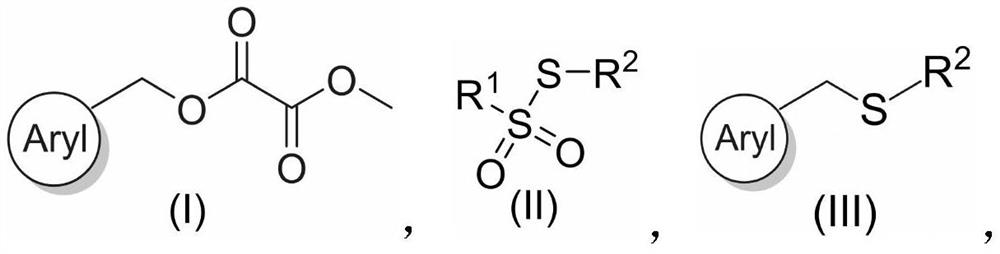
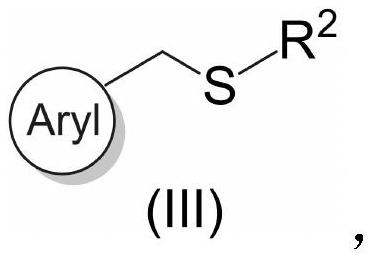
Benzyl thioether compound and preparation method thereof
A technology for benzylic thioethers and compounds, which is applied in the field of benzylic thioethers and their preparations, can solve the problems of strong irritation and toxicity of mercaptans, high cost, etc., and achieve high yield, simple raw materials, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of benzylic sulfide compound (naphthalene-2-ylmethyl) (phenyl) sulfide and preparation method thereof, the specific steps are as follows:
[0035]
[0036] In the glove box, an oven-dried screw cap 8 mL vial equipped with a magnetic stir bar was charged with methyl (naphthalen-2-ylmethyl)oxalate (0.2 mmol), thiosulfonate (0.24 mmol) and NiBr via syringe 2 (5.0 mol%), ligand 1,10-phenanthroline (10 mol%), Mn (1.5 equiv.), DMSO (1 mL), and the mixture was stirred at 40° C. for 24 h. After 24 h, the crude reaction mixture was diluted with ethyl acetate (20 mL) and washed with water (20 mL x 3). Na for organic layer 2 SO 4 Dry, filter and concentrate. The residue was purified by flash chromatography to give the product in 69% yield.
[0037] The compound prepared in this embodiment is detected, and the detection results are as follows:
[0038] Infrared spectrum (neat, ν, cm-1): 2953, 2921, 2852, 1730, 1477, 1438, 1270, 1089, 1022, 776, 686, 453, 470;
[003...
Embodiment 2
[0043]A benzylic sulfide compound ((6-methoxynaphthalene-2-yl) methyl) (phenyl) sulfide and a preparation method thereof, the specific steps are as follows:
[0044]
[0045] In a glove box, an oven-dried screw cap 8 mL vial equipped with a magnetic stir bar was charged with methyl (6-methoxynaphthalen-2-yl)methyl oxalate (0.2 mmol), thiosulfonate (0.24 mmol ) by adding NiBr via syringe 2 (5.0 mol%), ligand 1,10-phenanthroline (10 mol%), Mn (1.5 equiv.), DMSO (1 mL), and the mixture was stirred at 40° C. for 24 h. After 24 h, the crude reaction mixture was diluted with ethyl acetate (20 mL) and washed with water (20 mL x 3). Na for organic layer 2 SO 4 Dry, filter and concentrate. The residue was purified by flash chromatography to give the product in 67% yield.
[0046] The compound prepared in this embodiment is detected, and the detection results are as follows:
[0047] Infrared spectrum (neat, ν, cm-1): 2920, 2850, 2361, 1599, 1435, 1261, 1163, 1024, 856, 814, 73...
Embodiment 3
[0052] A kind of benzylic sulfide compound 8-((phenylthio) methyl) quinoline and preparation method thereof, the specific steps are as follows:
[0053]
[0054] In a glove box, an oven-dried screw cap 8 mL vial equipped with a magnetic stir bar was charged with methyl(quinolin-8-ylmethyl)oxalate (0.2 mmol), thiosulfonate (0.24 mmol) by Syringe added NiBr 2 (5.0 mol%), ligand 1,10-phenanthroline (10 mol%), Mn (1.5 equiv.), DMSO (1 mL), and the mixture was stirred at 40° C. for 24 h. After 24 h, the crude reaction mixture was diluted with ethyl acetate (20 mL) and washed with water (20 mL x 3). Na for organic layer 2 SO 4 Dry, filter and concentrate. The residue was purified by flash chromatography to give the product in 63% yield.
[0055] The compound prepared in this embodiment is detected, and the detection results are as follows:
[0056] Infrared spectrum (neat, ν, cm-1): 2921, 2853, 1725, 1573, 1472, 1272, 1071, 793, 728, 688;
[0057] Proton NMR spectrum (400M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com