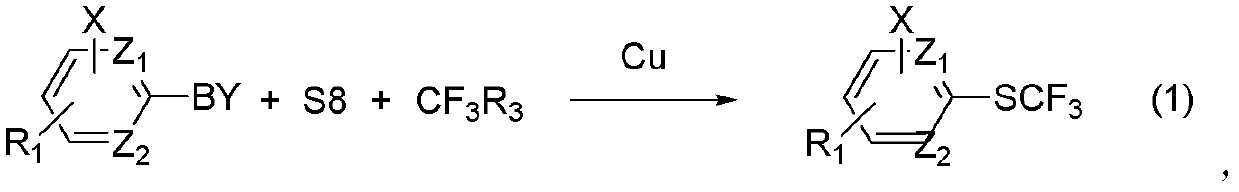
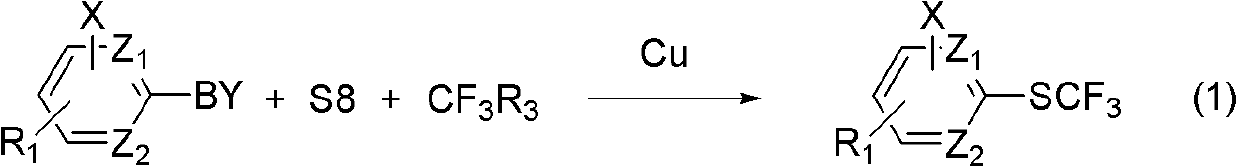
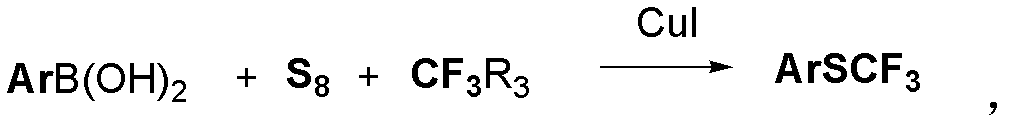Method for synthesizing aryl trifluoromethyl sulphydryl compound
A synthesis method, trifluoromethyl technology, applied in the field of synthesis of trifluoromethyl mercapto compounds, can solve the problems of high application cost and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Coupling reaction of phenylboronic acid, elemental sulfur and trifluoromethyltrimethylsilane under the catalysis of monovalent copper.
[0031]
[0032] Using N,N-dimethylformamide (5ml) as solvent, add phenylboronic acid (0.2), elemental sulfur (0.6mmol), and trifluoromethyltrimethylsilane (1.0-mmol) at any monovalent Stir at room temperature for 24 hours under the catalysis of a copper catalyst such as cuprous iodide (0.02 mmol). pass 19F NMR followed the reaction until completion. After removal of the solvent, column chromatography or distillation gave the corresponding aryl trifluoromethyl sulfide. Yield 45%. The relevant data are as follows: 1 H NMR (300MHz, CDCl3, 293K, TMS): δppm 7.35-7.39 (m, 4H), 7.23 (t, J=8.4Hz, 1H). 19 F NMR (282MHz, CDCl3): δppm-42.81(s, 3F). 13 C NMR (100.7MHz, CDCl3, 293K, TMS): δppm 166.04, 129.53 (d, J=0.8Hz), 128.93, 125.40, 36.46. IR (ATR): vmax 2955.49, 1436.91, 1284.86, 1160.71, 1017.51, 764.68 , 693.19cm -1 .M...
Embodiment 2
[0033] Example 2: Coupling reaction of phenylboronic acid, elemental sulfur and trifluoromethyltrimethylsilane under the catalysis of monovalent copper and organic ligands.
[0034]
[0035] Using N,N-dimethylformamide (5ml) as solvent, add phenylboronic acid (0.2mmol), elemental sulfur (0.6mmol), additive potassium phosphate (0.4mmol) and silver carbonate (0.4mmol) (without the additive) lead to a decrease in yield, and the use of other oxidants and bases also led to a certain decrease in yield), and trifluoromethyltrimethylsilane (1.0-mmol) in 1,10-phenanthroline (phen, 0.04mmol) Under the catalysis of the catalyst composed of CuSCN (0.02 mmol), stir at 25 ° C (parallel experiments show that the yield changes little between the reaction temperature of 0-50 ° C, and the reaction temperature exceeds this range, resulting in a decrease in yield) for 24 Hour( 19 F NMR tracking revealed comparable product formation after 1 hour). pass 19 F NMR followed the reaction until co...
Embodiment 3
[0036] Example 3: Coupling reaction of phenylboron tetrafluoride, elemental sulfur and trifluoromethyltriethylsilane under the catalysis of monovalent copper and organic ligands.
[0037]
[0038] Using N,N-dimethylformamide (5ml) as solvent, add phenylboron tetrafluoride (0.2mmol), elemental sulfur (0.6mmol), potassium phosphate (0.4mmol) and silver carbonate (0.4mmol), and Trifluoromethyltriethylsilane (1.0-mmol) was stirred at room temperature for 24 hours under the catalysis of a catalyst consisting of 1,10-phenanthroline (phen, 0.04 mmol) and CuSCN (0.02 mmol). After removal of the solvent, column chromatography or distillation gave the corresponding aryl trifluoromethyl sulfide. The yield was 89%, and it was found through experiments that phenylboronic esters also had similar reactivity in this reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com