Synthetic method of aryl sulfide type compound
A synthetic method and technology of aryl sulfide, applied in the formation/introduction of mercapto/thioether groups, chemical instruments and methods, thioether preparation, etc., can solve problems such as poor controllability, serious environmental pollution, and low compatibility , to achieve the effects of mild reaction conditions, simple reaction operation, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
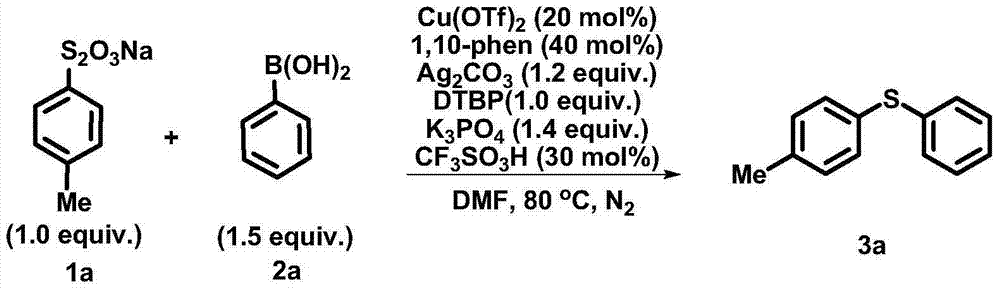
[0024] Synthesis of phenyl (4-methylphenyl) sulfide:
[0025]
[0026] Under nitrogen atmosphere, add substrate 1a (0.1mmol, 22.6mg), 2a (0.15mmol, 18.3mg), Cu(OTf) in a 25mL test tube reactor 2 (0.02mmol, 7.2mg), 1,10-phenanthroline (0.04mmol, 7.2mg), Ag 2 CO 3 (0.12mmol, 33.1mg), K 3 PO 4 (0.14mmol, 29.7mg), DTBP (0.1mmol, 18.5μL), CF 3 SO 3 H (3.0 μL), and DMF (2.0 mL). The reaction system was heated to 80°C for reaction. After the reaction was detected by TLC, the system was cooled to room temperature. The reaction was quenched with saturated aqueous ammonium chloride, extracted with ethyl acetate (3*10 mL), and purified by column chromatography to obtain product 3a (40%). R f =0.80 (petroleum ether); 1 H NMR (400MHz, CDCl3): δ7.18-7.24(m, 6H), 7.11-7.13(m, 1H), 7.06(d, J=8.0Hz, 2H), 2.27(s, 3H); 13C NMR (400MHz, CDCl3): δ137.6, 137.1, 132.3, 131.3, 130.0, 129.8, 129.0, 126.4, 21.1; IR (neat) 2950, 2851, 1896, 1492, 1477, 808, 739, 690cm -1 ; HRMS (EI) cal...
Embodiment 2
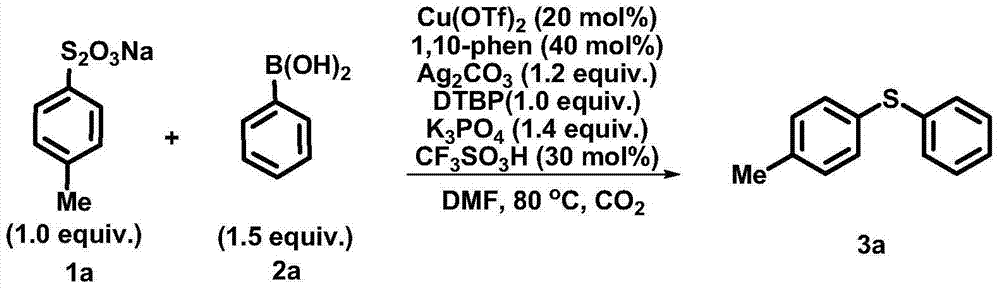
[0028] Synthesis of phenyl (4-methylphenyl) sulfide:
[0029]
[0030] Under carbon dioxide atmosphere, add substrate 1a (0.1mmol, 22.6mg), 2a (0.15mmol, 18.3mg), Cu(OTf) into a 25mL test tube reactor 2 (0.02mmol, 7.2mg), 1,10-phenanthroline (0.04mmol, 7.2mg), Ag 2 CO 3 (0.12mmol, 33.1mg), K 3 PO 4 (0.14mmol, 29.7mg), DTBP (0.1mmol, 18.5μL), CF 3 SO 3 H (3.0 μL), and DMF (2.0 mL). The reaction system was heated to 80°C for reaction. After the reaction was detected by TLC, the system was cooled to room temperature. The reaction was quenched with saturated aqueous ammonium chloride, extracted with ethyl acetate (3*10 mL), and purified by column chromatography to obtain product 3a (85%). The product data is the same as Example 1.
Embodiment 3
[0032] Synthesis of phenyl (4-methylphenyl) sulfide:
[0033]
[0034] Under carbon dioxide atmosphere, add substrate 1a (0.1mmol, 22.6mg), 2a (0.15mmol, 18.3mg), Cu(OTf) into a 25mL test tube reactor 2 (0.02mmol, 7.2mg), 1,10-phenanthroline (0.04mmol, 7.2mg), Ag 2 CO 3 (0.12mmol, 33.1mg), K 3 PO 4 (0.14 mmol, 29.7 mg), DTBP (0.1 mmol, 18.5 μL), and DMF (2.0 mL). The reaction system was heated to 80°C for reaction. After the reaction was detected by TLC, the system was cooled to room temperature. The reaction was quenched with saturated aqueous ammonium chloride, extracted with ethyl acetate (3*10 mL), and purified by column chromatography to obtain product 3a (67%). The product data is the same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com