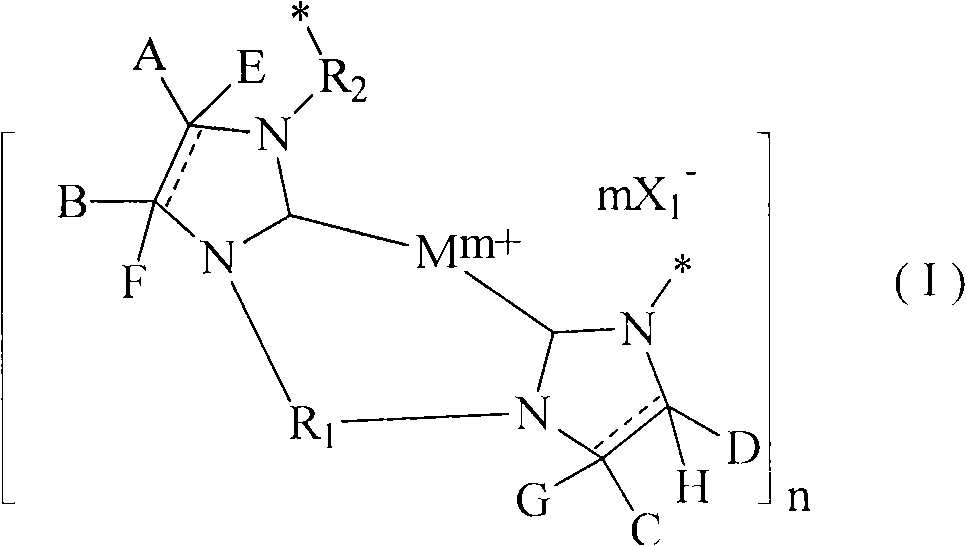
Poly-N-heterocyclic carbene transition metal complexes and N-heterocyclic carbene transition metal complexes for carbon-sulfur and carbon-oxygen coupling reactions
A transition metal, heterocyclic carbene technology, applied in the formation/introduction of nickel organic compounds, sulfhydryl/thioether group, formation/introduction of ether group/acetal group/ketal group, etc., can solve the problem of low activity and catalyst. Problems such as low loading and many synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0081] All solvents purchased from commercial suppliers were used as received unless otherwise stated. in Eppendorf TM Centrifugal separation was performed on a centrifuge 5810R (4000rpm, 10min). Using an Agilent equipped with a split sampling capillary injection system and a flame ionization detector TM The 6890N Series Gas Chromatograph performs gas-liquid chromatography. Using Shimadzu TM GCMS QP2010 for gas chromatography-mass spectrometry (GC-MS) analysis. Use ELAN TM 9000 / DRC system for inductively coupled plasma mass spectrometry (ICP-MS) analysis. The progress of the catalytic reaction can usually be monitored by GC or GC-MS analysis of a reaction sample.
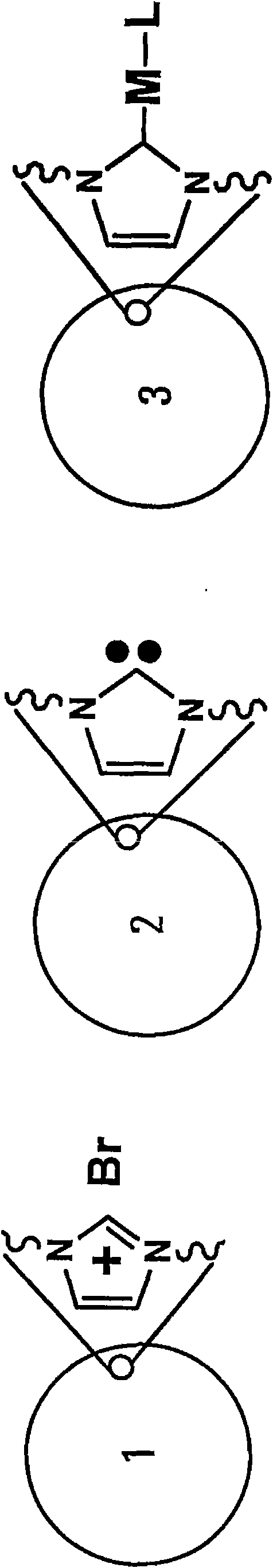
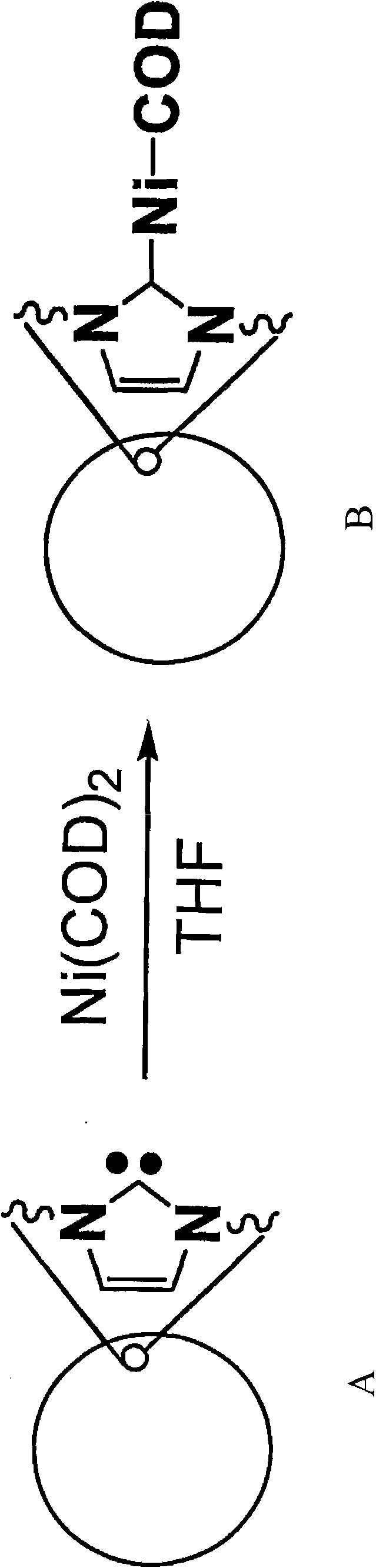
[0082] Synthesis of Ni(0)-p-NHC Catalyst
[0083] In the glove box, 82.5 mg of Ni(COD) 2(COD = cyclooctadiene) (0.3 mmol) was added to a THF suspension of polyimidazocarbene (p-Im) A (1 g). The mixture was stirred at room temperature for 16 hours. The suspension was then filtered and washed with DMF (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com